O-linked N-acetylglucosamine modification is essential for physiological adipose expansion induced by high-fat feeding
- PMID: 37224467
- PMCID: PMC10292976
- DOI: 10.1152/ajpendo.00263.2022
O-linked N-acetylglucosamine modification is essential for physiological adipose expansion induced by high-fat feeding
Abstract
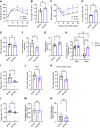
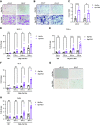
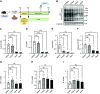
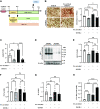
Adipose tissues accumulate excess energy as fat and heavily influence metabolic homeostasis. O-linked N-acetylglucosamine (O-GlcNAc) modification (O-GlcNAcylation), which involves the addition of N-acetylglucosamine to proteins by O-GlcNAc transferase (Ogt), modulates multiple cellular processes. However, little is known about the role of O-GlcNAcylation in adipose tissues during body weight gain due to overnutrition. Here, we report on O-GlcNAcylation in mice with high-fat diet (HFD)-induced obesity. Mice with knockout of Ogt in adipose tissue achieved using adiponectin promoter-driven Cre recombinase (Ogt-FKO) gained less body weight than control mice under HFD. Surprisingly, Ogt-FKO mice exhibited glucose intolerance and insulin resistance, despite their reduced body weight gain, as well as decreased expression of de novo lipogenesis genes and increased expression of inflammatory genes, resulting in fibrosis at 24 weeks of age. Primary cultured adipocytes derived from Ogt-FKO mice showed decreased lipid accumulation. Both primary cultured adipocytes and 3T3-L1 adipocytes treated with OGT inhibitor showed increased secretion of free fatty acids. Medium derived from these adipocytes stimulated inflammatory genes in RAW 264.7 macrophages, suggesting that cell-to-cell communication via free fatty acids might be a cause of adipose inflammation in Ogt-FKO mice. In conclusion, O-GlcNAcylation is important for healthy adipose expansion in mice. Glucose flux into adipose tissues may be a signal to store excess energy as fat.NEW & NOTEWORTHY We evaluated the role of O-GlcNAcylation in adipose tissue in diet-induced obesity using adipose tissue-specific Ogt knockout mice. We found that O-GlcNAcylation in adipose tissue is essential for healthy fat expansion and that Ogt-FKO mice exhibit severe fibrosis upon long-term overnutrition. O-GlcNAcylation in adipose tissue may regulate de novo lipogenesis and free fatty acid efflux to the degree of overnutrition. We believe that these results provide new insights into adipose tissue physiology and obesity research.
Keywords: O-GlcNAcylation; adipose inflammation; insulin resistance; leptin; obesity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures





References
-
- Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281: 40236–40241, 2006. doi:10.1074/jbc.M608048200. - DOI - PubMed
-
- Virtanen KA, Lönnroth P, Parkkola R, Peltoniemi P, Asola M, Viljanen T, Tolvanen T, Knuuti J, Rönnemaa T, Huupponen R, Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J Clin Endocrinol Metab 87: 3902–3910, 2002. doi:10.1210/jcem.87.8.8761. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

