A high-fat diet increases hepatic mitochondrial turnover through restricted acetylation in a NAFLD mouse model
- PMID: 37224468
- PMCID: PMC10312330
- DOI: 10.1152/ajpendo.00310.2022
A high-fat diet increases hepatic mitochondrial turnover through restricted acetylation in a NAFLD mouse model
Abstract
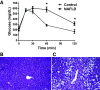
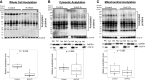
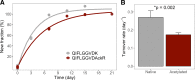
Lysine acetylation of proteins has emerged as a key posttranslational modification (PTM) that regulates mitochondrial metabolism. Acetylation may regulate energy metabolism by inhibiting and affecting the stability of metabolic enzymes and oxidative phosphorylation (OxPhos) subunits. Although protein turnover can be easily measured, due to the low abundance of modified proteins, it has been difficult to evaluate the effect of acetylation on the stability of proteins in vivo. We applied 2H2O-metabolic labeling coupled with immunoaffinity and high-resolution mass spectrometry method to measure the stability of acetylated proteins in mouse liver based on their turnover rates. As a proof-of-concept, we assessed the consequence of high-fat diet (HFD)-induced altered acetylation in protein turnover in LDL receptor-deficient (LDLR-/-) mice susceptible to diet-induced nonalcoholic fatty liver disease (NAFLD). HFD feeding for 12 wk led to steatosis, the early stage of NAFLD. A significant reduction in acetylation of hepatic proteins was observed in NAFLD mice, based on immunoblot analysis and label-free quantification with mass spectrometry. Compared with control mice on a normal diet, NAFLD mice had overall increased turnover rates of hepatic proteins, including mitochondrial metabolic enzymes (0.159 ± 0.079 vs. 0.132 ± 0.068 day-1), suggesting their reduced stability. Also, acetylated proteins had slower turnover rates (increased stability) than native proteins in both groups (0.096 ± 0.056 vs. 0.170 ± 0.059 day-1 in control, and 0.111 ± 0.050 vs. 0.208 ± 0.074 day-1 in NAFLD). Furthermore, association analysis revealed a relationship between the HFD-induced decrease in acetylation and increased turnover rates for hepatic proteins in NAFLD mice. These changes were associated with increased expressions of the hepatic mitochondrial transcriptional factor (TFAM) and complex II subunit without any changes to other OxPhos proteins, suggesting that enhanced mitochondrial biogenesis prevented restricted acetylation-mediated depletion of mitochondrial proteins. We conclude that decreased acetylation of mitochondrial proteins may contribute to adaptive improved hepatic mitochondrial function in the early stages of NAFLD.NEW & NOTEWORTHY This is the first method to quantify acetylome dynamics in vivo. This method revealed acetylation-mediated altered hepatic mitochondrial protein turnover in response to a high-fat diet in a mouse model of NAFLD.
Keywords: NAFLD; acetylome dynamics; metabolic labeling; mitochondria; turnover.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures







References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

