Targeted tissue delivery of RNA therapeutics using antibody-oligonucleotide conjugates (AOCs)
- PMID: 37224533
- PMCID: PMC10325888
- DOI: 10.1093/nar/gkad415
Targeted tissue delivery of RNA therapeutics using antibody-oligonucleotide conjugates (AOCs)
Abstract
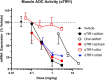
Although targeting TfR1 to deliver oligonucleotides to skeletal muscle has been demonstrated in rodents, effectiveness and pharmacokinetic/pharmacodynamic (PKPD) properties remained unknown in higher species. We developed antibody-oligonucleotide conjugates (AOCs) towards mice or monkeys utilizing anti-TfR1 monoclonal antibodies (αTfR1) conjugated to various classes of oligonucleotides (siRNA, ASOs and PMOs). αTfR1 AOCs delivered oligonucleotides to muscle tissue in both species. In mice, αTfR1 AOCs achieved a > 15-fold higher concentration to muscle tissue than unconjugated siRNA. A single dose of an αTfR1 conjugated to an siRNA against Ssb mRNA produced > 75% Ssb mRNA reduction in mice and monkeys, and mRNA silencing was greatest in skeletal and cardiac (striated) muscle with minimal to no activity in other major organs. In mice the EC50 for Ssb mRNA reduction in skeletal muscle was >75-fold less than in systemic tissues. Oligonucleotides conjugated to control antibodies or cholesterol produced no mRNA reduction or were 10-fold less potent, respectively. Tissue PKPD of AOCs demonstrated mRNA silencing activity primarily driven by receptor-mediated delivery in striated muscle for siRNA oligonucleotides. In mice, we show that AOC-mediated delivery is operable across various oligonucleotide modalities. AOC PKPD properties translated to higher species, providing promise for a new class of oligonucleotide therapeutics.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

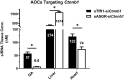




Comment in
-
Extrahepatic oligonucleotide delivery.Nat Rev Drug Discov. 2023 Aug;22(8):623. doi: 10.1038/d41573-023-00109-6. Nat Rev Drug Discov. 2023. PMID: 37400709 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

