Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism
- PMID: 37225143
- PMCID: PMC10681664
- DOI: 10.1093/cvr/cvad082
Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism
Erratum in
-
Corrigendum to: Macrophage angiotensin-converting enzyme reduces atherosclerosis by increasing peroxisome proliferator-activated receptor α and fundamentally changing lipid metabolism.Cardiovasc Res. 2024 Jul 2;120(8):971. doi: 10.1093/cvr/cvae013. Cardiovasc Res. 2024. PMID: 38301662 Free PMC article. No abstract available.
Abstract
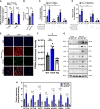
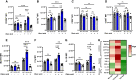
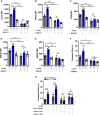
Aims: The metabolic failure of macrophages to adequately process lipid is central to the aetiology of atherosclerosis. Here, we examine the role of macrophage angiotensin-converting enzyme (ACE) in a mouse model of PCSK9-induced atherosclerosis.
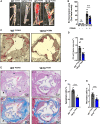
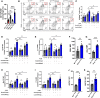
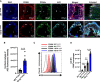
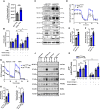
Methods and results: Atherosclerosis in mice was induced with AAV-PCSK9 and a high-fat diet. Animals with increased macrophage ACE (ACE 10/10 mice) have a marked reduction in atherosclerosis vs. WT mice. Macrophages from both the aorta and peritoneum of ACE 10/10 express increased PPARα and have a profoundly altered phenotype to process lipids characterized by higher levels of the surface scavenger receptor CD36, increased uptake of lipid, increased capacity to transport long chain fatty acids into mitochondria, higher oxidative metabolism and lipid β-oxidation as determined using 13C isotope tracing, increased cell ATP, increased capacity for efferocytosis, increased concentrations of the lipid transporters ABCA1 and ABCG1, and increased cholesterol efflux. These effects are mostly independent of angiotensin II. Human THP-1 cells, when modified to express more ACE, increase expression of PPARα, increase cell ATP and acetyl-CoA, and increase cell efferocytosis.
Conclusion: Increased macrophage ACE expression enhances macrophage lipid metabolism, cholesterol efflux, efferocytosis, and it reduces atherosclerosis. This has implications for the treatment of cardiovascular disease with angiotensin II receptor antagonists vs. ACE inhibitors.
Keywords: PPARα; angiotensin converting enzyme; atherosclerosis; lipid metabolism; macrophages.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: None declared.
Figures



References
-
- Chen R, Suchard MA, Krumholz HM, Schuemie MJ, Shea S, Duke J, Pratt N, Reich CG, Madigan D, You SC, Ryan PB, Hripcsak G. Comparative first-line effectiveness and safety of ACE (angiotensin-converting enzyme) inhibitors and angiotensin receptor blockers: a multinational cohort study. Hypertension 2021;78:591–603. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

