Cyclic processes in the uterine tubes, endometrium, myometrium, and cervix: pathways and perturbations
- PMID: 37225518
- PMCID: PMC10208902
- DOI: 10.1093/molehr/gaad012
Cyclic processes in the uterine tubes, endometrium, myometrium, and cervix: pathways and perturbations
Abstract
This review leads the 2023 Call for Papers in MHR: 'Cyclical function of the female reproductive tract' and will outline the complex and fascinating changes that take place in the reproductive tract during the menstrual cycle. We will also explore associated reproductive tract abnormalities that impact or are impacted by the menstrual cycle. Between menarche and menopause, women and people who menstruate living in high-income countries can expect to experience ∼450 menstrual cycles. The primary function of the menstrual cycle is to prepare the reproductive system for pregnancy in the event of fertilization. In the absence of pregnancy, ovarian hormone levels fall, triggering the end of the menstrual cycle and onset of menstruation. We have chosen to exclude the ovaries and focus on the other structures that make up the reproductive tract: uterine tubes, endometrium, myometrium, and cervix, which also functionally change in response to fluctuations in ovarian hormone production across the menstrual cycle. This inaugural paper for the 2023 MHR special collection will discuss our current understanding of the normal physiological processes involved in uterine cyclicity (limited specifically to the uterine tubes, endometrium, myometrium, and cervix) in humans, and other mammals where relevant. We will emphasize where knowledge gaps exist and highlight the impact that reproductive tract and uterine cycle perturbations have on health and fertility.
Keywords: cervix; endometrium; fallopian tube; fertility; menstrual cycle; menstruation; myometrium; uterine tube.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
The authors have no conflicts of interest to declare.
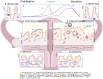
Figures
References
-
- Abberton KM, Healy DL, Rogers PA.. Smooth muscle alpha actin and myosin heavy chain expression in the vascular smooth muscle cells surrounding human endometrial arterioles. Hum Reprod 1999a;14:3095–3100. - PubMed
-
- Abberton KM, Taylor NH, Healy DL, Rogers PA.. Vascular smooth muscle cell proliferation in arterioles of the human endometrium. Hum Reprod 1999b;14:1072–1079. - PubMed
-
- Aguilar HN, Mitchell BF.. Physiological pathways and molecular mechanisms regulating uterine contractility. Hum Reprod Update 2010;16:725–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

