Phospholipase D3 degrades mitochondrial DNA to regulate nucleotide signaling and APP metabolism
- PMID: 37225734
- PMCID: PMC10209153
- DOI: 10.1038/s41467-023-38501-w
Phospholipase D3 degrades mitochondrial DNA to regulate nucleotide signaling and APP metabolism
Abstract
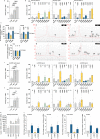
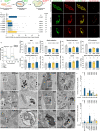
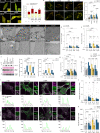
Phospholipase D3 (PLD3) polymorphisms are linked to late-onset Alzheimer's disease (LOAD). Being a lysosomal 5'-3' exonuclease, its neuronal substrates remained unknown as well as how a defective lysosomal nucleotide catabolism connects to AD-proteinopathy. We identified mitochondrial DNA (mtDNA) as a major physiological substrate and show its manifest build-up in lysosomes of PLD3-defective cells. mtDNA accretion creates a degradative (proteolytic) bottleneck that presents at the ultrastructural level as a marked abundance of multilamellar bodies, often containing mitochondrial remnants, which correlates with increased PINK1-dependent mitophagy. Lysosomal leakage of mtDNA to the cytosol activates cGAS-STING signaling that upregulates autophagy and induces amyloid precursor C-terminal fragment (APP-CTF) and cholesterol accumulation. STING inhibition largely normalizes APP-CTF levels, whereas an APP knockout in PLD3-deficient backgrounds lowers STING activation and normalizes cholesterol biosynthesis. Collectively, we demonstrate molecular cross-talks through feedforward loops between lysosomal nucleotide turnover, cGAS-STING and APP metabolism that, when dysregulated, result in neuronal endolysosomal demise as observed in LOAD.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

