A median fin derived from the lateral plate mesoderm and the origin of paired fins
- PMID: 37225983
- PMCID: PMC10266977
- DOI: 10.1038/s41586-023-06100-w
A median fin derived from the lateral plate mesoderm and the origin of paired fins
Abstract
The development of paired appendages was a key innovation during evolution and facilitated the aquatic to terrestrial transition of vertebrates. Largely derived from the lateral plate mesoderm (LPM), one hypothesis for the evolution of paired fins invokes derivation from unpaired median fins via a pair of lateral fin folds located between pectoral and pelvic fin territories1. Whilst unpaired and paired fins exhibit similar structural and molecular characteristics, no definitive evidence exists for paired lateral fin folds in larvae or adults of any extant or extinct species. As unpaired fin core components are regarded as exclusively derived from paraxial mesoderm, any transition presumes both co-option of a fin developmental programme to the LPM and bilateral duplication2. Here, we identify that the larval zebrafish unpaired pre-anal fin fold (PAFF) is derived from the LPM and thus may represent a developmental intermediate between median and paired fins. We trace the contribution of LPM to the PAFF in both cyclostomes and gnathostomes, supporting the notion that this is an ancient trait of vertebrates. Finally, we observe that the PAFF can be bifurcated by increasing bone morphogenetic protein signalling, generating LPM-derived paired fin folds. Our work provides evidence that lateral fin folds may have existed as embryonic anlage for elaboration to paired fins.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


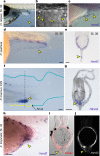

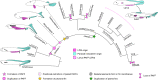

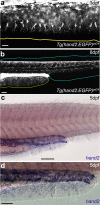

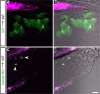




References
-
- Coates MI. The evolution of paired fins. Theory Biosci. 2003;122:266–287. doi: 10.1007/s12064-003-0057-4. - DOI
-
- Gegenbaur, C. Elements of Comparative Anatomy (Macmillan and Company, 1878).
-
- Balfour FM. On the development of the skeleton of the paired fins of Elasmobranchii, considered in relation to its bearings on the nature of the limbs of the Vertebrata. Proc. Zool. Soc. Lond. 1881;49:656–670. doi: 10.1111/j.1096-3642.1881.tb01323.x. - DOI
-
- Mivart SG. Notes on the fins of elasmobranchs, with considerations on the nature and homologues of vertebrate limbs. Trans. Zool. Soc. Lond. 1879;10:439–484. doi: 10.1111/j.1096-3642.1879.tb00460.x. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

