First-in-human, double-blind, randomized phase 1b study of peptide immunotherapy IMCY-0098 in new-onset type 1 diabetes
- PMID: 37226224
- PMCID: PMC10210318
- DOI: 10.1186/s12916-023-02900-z
First-in-human, double-blind, randomized phase 1b study of peptide immunotherapy IMCY-0098 in new-onset type 1 diabetes
Abstract
Background: Type 1 diabetes (T1D) is a CD4+ T cell-driven autoimmune disease characterized by the destruction of insulin-producing pancreatic β-cells by CD8+ T cells. Achieving glycemic targets in T1D remains challenging in clinical practice; new treatments aim to halt autoimmunity and prolong β-cell survival. IMCY-0098 is a peptide derived from human proinsulin that contains a thiol-disulfide oxidoreductase motif at the N-terminus and was developed to halt disease progression by promoting the specific elimination of pathogenic T cells.
Methods: This first-in-human, 24-week, double-blind phase 1b study evaluated the safety of three dosages of IMCY-0098 in adults diagnosed with T1D < 6 months before study start. Forty-one participants were randomized to receive four bi-weekly injections of placebo or increasing doses of IMCY-0098 (dose groups A/B/C received 50/150/450 μg for priming followed by three further administrations of 25/75/225 μg, respectively). Multiple T1D-related clinical parameters were also assessed to monitor disease progression and inform future development. Long-term follow-up to 48 weeks was also conducted in a subset of patients.
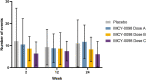
Results: Treatment with IMCY-0098 was well tolerated with no systemic reactions; a total of 315 adverse events (AEs) were reported in 40 patients (97.6%) and were related to study treatment in 29 patients (68.3%). AEs were generally mild; no AE led to discontinuation of the study or death. No significant decline in C-peptide was noted from baseline to Week 24 for dose A, B, C, or placebo (mean change - 0.108, - 0.041, - 0.040, and - 0.012, respectively), suggesting no disease progression.
Conclusions: Promising safety profile and preliminary clinical response data support the design of a phase 2 study of IMCY-0098 in patients with recent-onset T1D.
Trial registration: IMCY-T1D-001: ClinicalTrials.gov NCT03272269; EudraCT: 2016-003514-27; and IMCY-T1D-002: ClinicalTrials.gov NCT04190693; EudraCT: 2018-003728-35.
Keywords: Beta-cells; Clinical study; Immunotherapy; Safety; T cells; Type 1 diabetes.
© 2023. The Author(s).
Conflict of interest statement
JVR, VC, RRA, EG, NB, LVE, MVM, and PV are employees or contractors of Imcyse S.A., Liège, Belgium, and may hold stock options. RDL’s institution received study funding and materials from Imcyse. RDL received an honorarium from DMRR and took part in advisory boards for Diamyd and Provention. MAA’s institution received study funding and materials from Imcyse. MAA received medical writing and APC support from Imcyse; received grants from EFSD, Wellcome Trust, Cardiff University, Wales Kidney Research Unit, and INNODIA; received honoraria from Sanofi Diabetes, Eli Lilly, Boehringer Ingelheim, Astra Zeneca, MSD, Novo Nordisk and Bayer; received meeting support from Sanofi Diabetes, Eli Lilly, Takeda, Abbott, Merck, Novo Nordisk, NAPP, Miltenyi Biotec and Servier. CD, BK, and CB’s institutions received study funding and materials from Imcyse. CD received consultancy honoraria, medical writing, and APC support from Imcyse. PA and KRO declare no competing interests.
Figures




References
-
- Global report on diabetes. World Health Organization [https://www.who.int/diabetes/global-report/en/].
-
- American Diabetes Association Professional Practice C. Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, et al. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Supplement_1):S17–S38. doi: 10.2337/dc22-S002. - DOI - PubMed
-
- National Diabetes Statistics Report. Center for disease control and prevention [https://www.cdc.gov/diabetes/data/statistics/statistics-report.html].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

