Hydrogen peroxide sensor HyPer7 illuminates tissue-specific plastid redox dynamics
- PMID: 37226328
- PMCID: PMC10702466
- DOI: 10.1093/plphys/kiad307
Hydrogen peroxide sensor HyPer7 illuminates tissue-specific plastid redox dynamics
Abstract
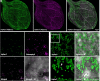
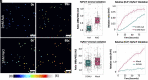
The visualization of photosynthesis-derived reactive oxygen species has been experimentally limited to pH-sensitive probes, unspecific redox dyes, and whole-plant phenotyping. Recent emergence of probes that circumvent these limitations permits advanced experimental approaches to investigate in situ plastid redox properties. Despite growing evidence of heterogeneity in photosynthetic plastids, investigations have not addressed the potential for spatial variation in redox and/or reactive oxygen dynamics. To study the dynamics of H2O2 in distinct plastid types, we targeted the pH-insensitive, highly specific probe HyPer7 to the plastid stroma in Arabidopsis (Arabidopsis thaliana). Using HyPer7 and glutathione redox potential (EGSH) probe for redox-active green fluorescent protein 2 genetically fused to the redox enzyme human glutaredoxin-1 with live cell imaging and optical dissection of cell types, we report heterogeneities in H2O2 accumulation and redox buffering within distinct epidermal plastids in response to excess light and hormone application. Our observations suggest that plastid types can be differentiated by their physiological redox features. These data underscore the variation in photosynthetic plastid redox dynamics and demonstrate the need for cell-type-specific observations in future plastid phenotyping.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



References
-
- Agarie S, Umemoto M, Sunagawa H, Anai T, Cushman JC. An Agrobacterium-mediated transformation via organogenesis regeneration of a facultative CAM plant, the common ice plant Mesembryanthemum crystallinum L. Plant Prod Sci. 2020:23(3):343–349. 10.1080/1343943X.2020.1730700 - DOI
-
- Azoulay-Shemer T, Palomares A, Bagheri A, Israelsson-Nordstrom M, Engineer CB, Bargmann BOR, Stephan AB, Schroeder JI. Guard cell photosynthesis is critical for stomatal turgor production, yet does not directly mediate CO2- and ABA-induced stomatal closing. Plant J. 2015:83(4):567–581. 10.1111/tpj.12916 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

