Aflibercept versus ranibizumab for diabetic macular edema: A meta-analysis
- PMID: 37226427
- PMCID: PMC11067396
- DOI: 10.1177/11206721231178658
Aflibercept versus ranibizumab for diabetic macular edema: A meta-analysis
Abstract
Objective: The purpose of this study was to compare the efficacy and safety of aflibercept (AFL) versus ranibizumab (RAN) for the treatment of diabetic macular edema (DME).
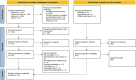
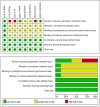
Methods: The PubMed, Embase, Cochrane Library, and CNKI databases were searched up to September 2022 to identify prospective randomized controlled trials (RCTs) comparing AFL with RAN for the treatment of DME. Review Manager 5.3 software was used for data analysis. We used the GRADE system to evaluate the quality of the evidence for each outcome.
Results: A total of 8 RCTs involving 1067 eyes (939 patients) were included; there were 526 eyes in the AFL group and 541 eyes in the RAN group. Meta-analysis revealed that there was no significant difference between RAN and AFL in the best-corrected visual acuity (BCVA) of DME patients at 6 months (WMD: -0.05, 95% CI = -0.12 to 0.01, moderate quality) and 12 months after injection (WMD: -0.02, 95% CI = -0.07 to 0.03, moderate quality). Additionally, there was no significant difference between RAN and AFL in the reduction of central macular thickness (CMT) at 6 months (WMD: -0.36, 95% CI = -24.99 to 24.26, very low quality) and 12 months after injection (WMD: -6.36, 95% CI = -16.30 to 3.59, low quality). Meta-analysis showed that the number of intravitreal injections (IVIs) for AFL was significantly lower than that for RAN (WMD: -0.47, 95% CI = -0.88 to -0.05, very low quality). There were fewer adverse reactions to AFL than to RAN, but the difference was not significant.
Conclusion: This study found that there was no difference in BCVA, CMT or adverse reactions between AFL and RAN at 6 and 12 months of follow-up, but AFL needed fewer IVIs than RAN.
Keywords: Aflibercept; diabetic macular edema; meta-analysis; ranibizumab.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


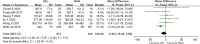
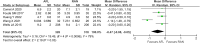
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

