Safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit COVID-19 vaccine in healthy 12-17 year-old adolescents
- PMID: 37226504
- PMCID: PMC10294745
- DOI: 10.1080/21645515.2023.2206359
Safety and immunogenicity of SCB-2019, an adjuvanted, recombinant SARS-CoV-2 trimeric S-protein subunit COVID-19 vaccine in healthy 12-17 year-old adolescents
Abstract
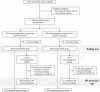
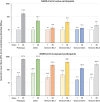
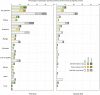
We previously demonstrated the efficacy of the COVID-19 vaccine candidate, SCB-2019, in adults in the SPECTRA phase 2/3 efficacy study. We extended the study to include 1278 healthy 12-17-year-old adolescents in Belgium, Colombia, and the Philippines who received either two doses of SCB-2019 or placebo 21 days apart, to assess immunogenicity as neutralizing antibodies against prototype SARS-CoV-2 and variants of concern, and safety and reactogenicity as solicited and unsolicited adverse events with a comparator group of young adults (18-25 years). In participants with no evidence of prior SARS-CoV-2 infection SCB-2019 immunogenicity in adolescents was non-inferior to that in young adults; respective geometric mean neutralizing titers (GMT) against prototype SARS-CoV-2 14 days after the second vaccination were 271 IU/mL (95% CI: 211-348) and 144 IU/mL (116-178). Most adolescents (1077, 84.3%) had serologic evidence of prior SAR-CoV-2 exposure at baseline; in these seropositive adolescents neutralizing GMTs increased from 173 IU/mL (135-122) to 982 IU/mL (881-1094) after the second dose. Neutralizing titers against Delta and Omicron BA SARS-CoV-2 variants were also increased, most notably in those with prior exposure. SCB-2019 vaccine was well tolerated with generally mild or moderate, transient solicited and unsolicited adverse events that were comparable in adolescent vaccine and placebo groups except for injection site pain - reported after 20% of SCB-2019 and 7.3% of placebo injections. SCB-2019 vaccine was highly immunogenic against SARS-CoV-2 prototype and variants in adolescents, especially in those with evidence of prior exposure, with comparable immunogenicity to young adults. Clinical trial registration: EudraCT 2020-004272-17; ClinicalTrials.gov NCT04672395.
Keywords: COVID-19; SCB-2019; adolescents; immunogenicity; reactogenicity; vaccine.
Conflict of interest statement
YH, BH, PL, HHH, CB, and IS are full-time employees of Clover Biopharmaceuticals. Other authors have no interest to declare.
Figures

Similar articles
-
Immunogenicity of an adjuvanted SARS-CoV-2 trimeric S-protein subunit vaccine (SCB-2019) in SARS-CoV-2-naïve and exposed individuals in a phase 2/3, double-blind, randomized study.Vaccine. 2023 Mar 10;41(11):1875-1884. doi: 10.1016/j.vaccine.2023.02.017. Epub 2023 Feb 9. Vaccine. 2023. PMID: 36781334 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial.Lancet. 2021 Feb 20;397(10275):682-694. doi: 10.1016/S0140-6736(21)00241-5. Epub 2021 Jan 29. Lancet. 2021. PMID: 33524311 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a protein subunit COVID-19 vaccine (ZF2001) in healthy children and adolescents aged 3-17 years in China: a randomised, double-blind, placebo-controlled, phase 1 trial and an open-label, non-randomised, non-inferiority, phase 2 trial.Lancet Child Adolesc Health. 2023 Apr;7(4):269-279. doi: 10.1016/S2352-4642(22)00376-5. Epub 2023 Feb 17. Lancet Child Adolesc Health. 2023. PMID: 36803632 Free PMC article. Clinical Trial.
-
Immunogenicity, efficacy, and safety of SARS-CoV-2 vaccine dose fractionation: a systematic review and meta-analysis.BMC Med. 2022 Oct 25;20(1):409. doi: 10.1186/s12916-022-02600-0. BMC Med. 2022. PMID: 36284331 Free PMC article.
-
An Outline of the Immunogenic Potential of Progressing SARSCoV- 2 Vaccine Technologies among Children and Adolescents.Recent Pat Biotechnol. 2024;18(3):180-189. doi: 10.2174/1872208317666230612141930. Recent Pat Biotechnol. 2024. PMID: 38528666 Review.
Cited by
-
A Review of Protein-Based COVID-19 Vaccines: From Monovalent to Multivalent Formulations.Vaccines (Basel). 2024 May 25;12(6):579. doi: 10.3390/vaccines12060579. Vaccines (Basel). 2024. PMID: 38932308 Free PMC article. Review.
-
Immunogenicity and safety of adjuvant-associated COVID-19 vaccines: A systematic review and meta-analysis of randomized controlled trials.Heliyon. 2023 Nov 28;9(12):e22858. doi: 10.1016/j.heliyon.2023.e22858. eCollection 2023 Dec. Heliyon. 2023. PMID: 38125524 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
