DNA methyltransferase inhibitor 5-azacytidine enhances neuroblastoma cell lysis by an oncolytic parainfluenza virus
- PMID: 37227036
- PMCID: PMC10414160
- DOI: 10.1097/CAD.0000000000001525
DNA methyltransferase inhibitor 5-azacytidine enhances neuroblastoma cell lysis by an oncolytic parainfluenza virus
Abstract
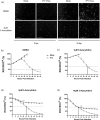
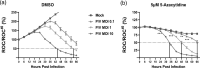
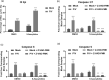
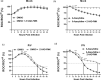
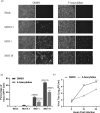
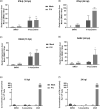
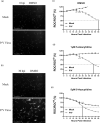
Studies with neuroblastoma have shown that the presence of aberrant DNA epigenetic modifications mediated by DNA methyltransferases correlates with poor prognosis, making these enzymes a target for therapeutics based on synthetic epigenetic modulators such as DNA methyltransferase inhibitors (DNMTi). Here, we have used a neuroblastoma cell line model to test the hypothesis that treatment with a DNMTi would enhance cell killing when used in combination with oncolytic Parainfluenza virus 5 (P/V virus), a cytoplasmic-replicating RNA virus. Pretreatment of SK-N-AS cells with the DNMTi 5-azacytidine substantially enhanced P/V virus-mediated cell death in a dose- and multiplicity of infection-dependent manner. Infection with the virus alone and the combination treatment with 5-azacytidine and P/V virus infection led to the activation of caspases-8, -9, and -3/7. Inhibition of caspases using a pan-caspase inhibitor minimally affected cell killing by P/V virus alone, but by contrast, largely reduced cell death mediated by 5-azacytidine treatment alone or in combination with P/V virus infection. 5-Azacytidine pretreatment dampened P/V virus gene expression and growth within the SK-N-AS cell population, which correlated with enhanced expression of important antiviral genes such as interferon-β and OAS2 . Taken together, our data support the role of combination treatment using 5-azacytidine and an oncolytic P/V virus for neuroblastoma therapy.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Histone Deacetylase Inhibitors Enhance Cell Killing and Block Interferon-Beta Synthesis Elicited by Infection with an Oncolytic Parainfluenza Virus.Viruses. 2019 May 10;11(5):431. doi: 10.3390/v11050431. Viruses. 2019. PMID: 31083335 Free PMC article.
-
5-Azacytidine, a DNA methyltransferase inhibitor, induces ATR-mediated DNA double-strand break responses, apoptosis, and synergistic cytotoxicity with doxorubicin and bortezomib against multiple myeloma cells.Mol Cancer Ther. 2007 Jun;6(6):1718-27. doi: 10.1158/1535-7163.MCT-07-0010. Mol Cancer Ther. 2007. PMID: 17575103
-
Impact of DNA methyltransferase inhibitor 5-azacytidine on cardiac development of zebrafish in vivo and cardiomyocyte proliferation, apoptosis, and the homeostasis of gene expression in vitro.J Cell Biochem. 2019 Oct;120(10):17459-17471. doi: 10.1002/jcb.29010. Epub 2019 Jul 4. J Cell Biochem. 2019. PMID: 31271227
-
Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine.Int J Cancer. 2008 Jul 1;123(1):8-13. doi: 10.1002/ijc.23607. Int J Cancer. 2008. PMID: 18425818 Review.
-
A Comparative Study on the In Vitro Effects of the DNA Methyltransferase Inhibitor 5-Azacytidine (5-AzaC) in Breast/Mammary Cancer of Different Mammalian Species.J Mammary Gland Biol Neoplasia. 2016 Jun;21(1-2):51-66. doi: 10.1007/s10911-016-9350-y. Epub 2016 Mar 22. J Mammary Gland Biol Neoplasia. 2016. PMID: 27002722 Review.
Cited by
-
Molecular biology of canine parainfluenza virus V protein and its potential applications in tumor immunotherapy.Front Microbiol. 2023 Dec 20;14:1282112. doi: 10.3389/fmicb.2023.1282112. eCollection 2023. Front Microbiol. 2023. PMID: 38173672 Free PMC article. Review.
-
Therapy-induced senescence in breast cancer: an overview.Explor Target Antitumor Ther. 2024;5(4):902-920. doi: 10.37349/etat.2024.00254. Epub 2024 Jul 25. Explor Target Antitumor Ther. 2024. PMID: 39280248 Free PMC article. Review.
-
Harnessing Epigenetics: Innovative Approaches in Diagnosing and Combating Viral Acute Respiratory Infections.Pathogens. 2025 Feb 1;14(2):129. doi: 10.3390/pathogens14020129. Pathogens. 2025. PMID: 40005506 Free PMC article. Review.
References
-
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, et al. . Neuroblastoma. Nat Rev Dis Primers 2016; 2:16078. - PubMed
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SLN. Neuroblastoma. Lancet 2007; 369:2106–2120. - PubMed
-
- Qiu YY, Mirkin BL, Dwivedi RS. Inhibition of DNA methyltransferase reverses cisplatin induced drug resistance in murine neuroblastoma cells. Cancer Detect Prev 2005; 29:456–463. - PubMed
-
- Christman JK. 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene 2002; 21:5483–5495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

