Phase I Study of SYNB1891, an Engineered E. coli Nissle Strain Expressing STING Agonist, with and without Atezolizumab in Advanced Malignancies
- PMID: 37227176
- PMCID: PMC11225568
- DOI: 10.1158/1078-0432.CCR-23-0118
Phase I Study of SYNB1891, an Engineered E. coli Nissle Strain Expressing STING Agonist, with and without Atezolizumab in Advanced Malignancies
Abstract
Purpose: SYNB1891 is a live, modified strain of the probiotic Escherichia coli Nissle 1917 (EcN) engineered to produce cyclic dinucleotides under hypoxia, leading to STimulator of INterferon Genes (STING) activation in phagocytic antigen-presenting cells in tumors and activating complementary innate immune pathways.
Patients and methods: This first-in-human study (NCT04167137) enrolled participants with refractory advanced cancers to receive repeat intratumoral injections of SYNB1891 either alone or in combination with atezolizumab, with the primary objective of evaluating the safety and tolerability of both regimens.
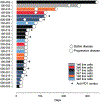
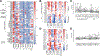
Results: Twenty-four participants received monotherapy across six cohorts, and 8 participants received combination therapy in two cohorts. Five cytokine release syndrome events occurred with monotherapy, including one that met the criteria for dose-limiting toxicity at the highest dose; no other SYNB1891-related serious adverse events occurred, and no SYNB1891-related infections were observed. SYNB1891 was not detected in the blood at 6 or 24 hours after the first intratumoral dose or in tumor tissue 7 days following the first dose. Treatment with SYNB1891 resulted in activation of the STING pathway and target engagement as assessed by upregulation of IFN-stimulated genes, chemokines/cytokines, and T-cell response genes in core biopsies obtained predose and 7 days following the third weekly dose. In addition, a dose-related increase in serum cytokines was observed, as well as stable disease in 4 participants refractory to prior PD-1/L1 antibodies.
Conclusions: Repeat intratumoral injection of SYNB1891 as monotherapy and in combination with atezolizumab was safe and well tolerated, and evidence of STING pathway target engagement was observed.
©2023 American Association for Cancer Research.
Figures

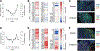
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

