Dynamics of SARS-CoV-2 seroassay sensitivity: a systematic review and modelling study
- PMID: 37227301
- PMCID: PMC10283460
- DOI: 10.2807/1560-7917.ES.2023.28.21.2200809
Dynamics of SARS-CoV-2 seroassay sensitivity: a systematic review and modelling study
Abstract
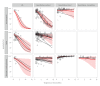
BackgroundSerological surveys have been the gold standard to estimate numbers of SARS-CoV-2 infections, the dynamics of the epidemic, and disease severity. Serological assays have decaying sensitivity with time that can bias their results, but there is a lack of guidelines to account for this phenomenon for SARS-CoV-2.AimOur goal was to assess the sensitivity decay of seroassays for detecting SARS-CoV-2 infections, the dependence of this decay on assay characteristics, and to provide a simple method to correct for this phenomenon.MethodsWe performed a systematic review and meta-analysis of SARS-CoV-2 serology studies. We included studies testing previously diagnosed, unvaccinated individuals, and excluded studies of cohorts highly unrepresentative of the general population (e.g. hospitalised patients).ResultsOf the 488 screened studies, 76 studies reporting on 50 different seroassays were included in the analysis. Sensitivity decay depended strongly on the antigen and the analytic technique used by the assay, with average sensitivities ranging between 26% and 98% at 6 months after infection, depending on assay characteristics. We found that a third of the included assays departed considerably from manufacturer specifications after 6 months.ConclusionsSeroassay sensitivity decay depends on assay characteristics, and for some types of assays, it can make manufacturer specifications highly unreliable. We provide a tool to correct for this phenomenon and to assess the risk of decay for a given assay. Our analysis can guide the design and interpretation of serosurveys for SARS-CoV-2 and other pathogens and quantify systematic biases in the existing serology literature.
Keywords: Antibody; Meta-analysis; SARS-CoV-2, COVID-19; Serological assays; Serology; Serosurveillance.
Conflict of interest statement
Figures


References
-
- Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35(12):1123-38. 10.1007/s10654-020-00698-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
