Functional Epicardial Conduction Disturbances Due to a SCN5A Variant Associated With Brugada Syndrome
- PMID: 37227351
- PMCID: PMC10406612
- DOI: 10.1016/j.jacep.2023.03.009
Functional Epicardial Conduction Disturbances Due to a SCN5A Variant Associated With Brugada Syndrome
Abstract
Background: Brugada syndrome is a significant cause of sudden cardiac death (SCD), but the underlying mechanisms remain hypothetical.
Objectives: This study aimed to elucidate this knowledge gap through detailed ex vivo human heart studies.
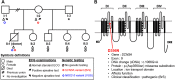
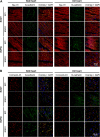
Methods: A heart was obtained from a 15-year-old adolescent boy with normal electrocardiogram who experienced SCD. Postmortem genotyping was performed, and clinical examinations were done on first-degree relatives. The right ventricle was optically mapped, followed by high-field magnetic resonance imaging and histology. Connexin-43 and NaV1.5 were localized by immunofluorescence, and RNA and protein expression levels were studied. HEK-293 cell surface biotinylation assays were performed to examine NaV1.5 trafficking.
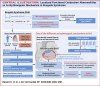
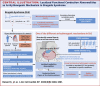
Results: A Brugada-related SCD diagnosis was established for the donor because of a SCN5A Brugada-related variant (p.D356N) inherited from his mother, together with a concomitant NKX2.5 variant of unknown significance. Optical mapping demonstrated a localized epicardial region of impaired conduction near the outflow tract, in the absence of repolarization alterations and microstructural defects, leading to conduction blocks and figure-of-8 patterns. NaV1.5 and connexin-43 localizations were normal in this region, consistent with the finding that the p.D356N variant does not affect the trafficking, nor the expression of NaV1.5. Trends of decreased NaV1.5, connexin-43, and desmoglein-2 protein levels were noted; however, the RT-qPCR results suggested that the NKX2-5 variant was unlikely to be involved.
Conclusions: This study demonstrates for the first time that SCD associated with a Brugada-SCN5A variant can be caused by localized functionally, not structurally, impaired conduction.
Keywords: Brugada syndrome; SCN5A variant; epicardial arrhythmogenic conduction substrate; right ventricle; sudden cardiac death.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work received financial support from the French Government as part of the “Investments of the Future” program managed by the National Research Agency (ANR-10-IAHU04-LIRYC), the Leducq-Foundation (RHYTHM network, 16CVD02), and the Fondation Coeur et Artères (FC17T2). Dr Barc is supported by the ANR JCJC LEARN (R21006NN, RPV21014NNA). Dr Schott is supported by IRP-, an I-SITE NExT health and engineering initiative (Ecole Centrale & Nantes University) and by the IRP- GAINES funded by INSERM and CNRS. Dr Marionneau is supported by the ANR-16-CE92-0013-01 and the National Institutes of Health (R01-HL148803). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures






Comment in
-
Delayed Activation Due to Early Repolarization: A Combination of Hypotheses.JACC Clin Electrophysiol. 2023 Aug;9(8 Pt 1):1262-1264. doi: 10.1016/j.jacep.2023.04.022. Epub 2023 Jun 21. JACC Clin Electrophysiol. 2023. PMID: 37354181 No abstract available.
-
The Mechanism of Brugada Syndrome: Is it Induced Only by Conduction Disturbance?JACC Clin Electrophysiol. 2023 Nov;9(11):2356. doi: 10.1016/j.jacep.2023.08.038. JACC Clin Electrophysiol. 2023. PMID: 38030334 No abstract available.
References
-
- Brugada J., Campuzano O., Arbelo E., Sarquella-Brugada G., Brugada R. Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(9):1046–1059. - PubMed
-
- Ten Sande J.N., Coronel R., Conrath C.E., Driessen A.H.G., de Groot J.R., Tan H.L., et al. ST-segment elevation and fractionated electrograms in Brugada syndrome patients arise from the same structurally abnormal subepicardial RVOT area but have a different mechanism. Circ Arrhythm Electrophysiol. 2015;8(6) - PubMed
-
- Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasiriojanakorn K., et al. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123(12):1270–12794. - PubMed
-
- Pieroni M., Notarstefano P., Oliva A., Campuzano O., Santangeli P., Coll M., et al. Electroanatomic and pathologic right ventricular outflow tract abnormalities in patients with Brugada syndrome. J Am Coll Cardiol. 2018;72 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

