Integrating contact tracing and whole-genome sequencing to track the elimination of dog-mediated rabies: An observational and genomic study
- PMID: 37227428
- PMCID: PMC10299823
- DOI: 10.7554/eLife.85262
Integrating contact tracing and whole-genome sequencing to track the elimination of dog-mediated rabies: An observational and genomic study
Abstract
Background: Dog-mediated rabies is endemic across Africa causing thousands of human deaths annually. A One Health approach to rabies is advocated, comprising emergency post-exposure vaccination of bite victims and mass dog vaccination to break the transmission cycle. However, the impacts and cost-effectiveness of these components are difficult to disentangle.
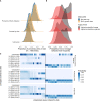
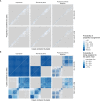
Methods: We combined contact tracing with whole-genome sequencing to track rabies transmission in the animal reservoir and spillover risk to humans from 2010 to 2020, investigating how the components of a One Health approach reduced the disease burden and eliminated rabies from Pemba Island, Tanzania. With the resulting high-resolution spatiotemporal and genomic data, we inferred transmission chains and estimated case detection. Using a decision tree model, we quantified the public health burden and evaluated the impact and cost-effectiveness of interventions over a 10-year time horizon.
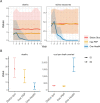
Results: We resolved five transmission chains co-circulating on Pemba from 2010 that were all eliminated by May 2014. During this period, rabid dogs, human rabies exposures and deaths all progressively declined following initiation and improved implementation of annual islandwide dog vaccination. We identified two introductions to Pemba in late 2016 that seeded re-emergence after dog vaccination had lapsed. The ensuing outbreak was eliminated in October 2018 through reinstated islandwide dog vaccination. While post-exposure vaccines were projected to be highly cost-effective ($256 per death averted), only dog vaccination interrupts transmission. A combined One Health approach of routine annual dog vaccination together with free post-exposure vaccines for bite victims, rapidly eliminates rabies, is highly cost-effective ($1657 per death averted) and by maintaining rabies freedom prevents over 30 families from suffering traumatic rabid dog bites annually on Pemba island.
Conclusions: A One Health approach underpinned by dog vaccination is an efficient, cost-effective, equitable, and feasible approach to rabies elimination, but needs scaling up across connected populations to sustain the benefits of elimination, as seen on Pemba, and for similar progress to be achieved elsewhere.
Funding: Wellcome [207569/Z/17/Z, 095787/Z/11/Z, 103270/Z/13/Z], the UBS Optimus Foundation, the Department of Health and Human Services of the National Institutes of Health [R01AI141712] and the DELTAS Africa Initiative [Afrique One-ASPIRE/DEL-15-008] comprising a donor consortium of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), the New Partnership for Africa's Development Planning and Coordinating (NEPAD) Agency, Wellcome [107753/A/15/Z], Royal Society of Tropical Medicine and Hygiene Small Grant 2017 [GR000892] and the UK government. The rabies elimination demonstration project from 2010-2015 was supported by the Bill & Melinda Gates Foundation [OPP49679]. Whole-genome sequencing was partially supported from APHA by funding from the UK Department for Environment, Food and Rural Affairs (Defra), Scottish government and Welsh government under projects SEV3500 and SE0421.
Keywords: epidemiology; global health; infectious disease; microbiology; next generation sequencing; one health; surveillance; vaccine-preventable; viruses; zoonotic disease.
© 2023, Lushasi, Brunker et al.
Conflict of interest statement
KL, KB, MR, EF, GJ, LB, RB, JC, SC, AC, AF, NG, DH, PJ, RK, TL, DM, MM, MM, EM, GM, AM, EM, CN, KN, HN, KO, KR, MS, LS, RS, KH No competing interests declared
Figures













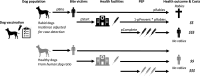
Update of
References
-
- Bourhy H, Nakouné E, Hall M, Nouvellet P, Lepelletier A, Talbi C, Watier L, Holmes EC, Cauchemez S, Lemey P, Donnelly CA, Rambaut A, Parrish C. Revealing the micro-scale signature of endemic zoonotic disease transmission in an African urban setting. PLOS Pathogens. 2016;12:e1005525. doi: 10.1371/journal.ppat.1005525. - DOI - PMC - PubMed
-
- Brunker K, Marston DA, Horton DL, Cleaveland S, Fooks AR, Kazwala R, Ngeleja C, Lembo T, Sambo M, Mtema ZJ, Sikana L, Wilkie G, Biek R, Hampson K. Elucidating the phylodynamics of endemic rabies virus in eastern Africa using whole-genome sequencing. Virus Evolution. 2015;1:vev011. doi: 10.1093/ve/vev011. - DOI - PMC - PubMed
-
- Brunker K, Jaswant G, Thumbi SM, Lushasi K, Lugelo A, Czupryna AM, Ade F, Wambura G, Chuchu V, Steenson R, Ngeleja C, Bautista C, Manalo DL, Gomez MRR, Chu M, Miranda ME, Kamat M, Rysava K, Espineda J, Silo EAV, Aringo AM, Bernales RP, Adonay FF, Tildesley MJ, Marston DA, Jennings DL, Fooks AR, Zhu W, Meredith LW, Hill SC, Poplawski R, Gifford RJ, Singer JB, Maturi M, Mwatondo A, Biek R, Hampson K. Rapid in-country sequencing of whole virus genomes to inform rabies elimination programmes. Wellcome Open Research. 2020;5:3. doi: 10.12688/wellcomeopenres.15518.2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

