The power of telemedicine to improve CAR-T cell therapy programs: lessons learned from COVID-19 pandemic
- PMID: 37227523
- PMCID: PMC10209549
- DOI: 10.1007/s00520-023-07811-6
The power of telemedicine to improve CAR-T cell therapy programs: lessons learned from COVID-19 pandemic
Abstract
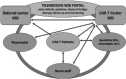
Purpose: CAR-T programs will burden increasingly on healthcare systems, since the implementation of these therapies involves: multidisciplinary team collaboration, post-infusion hospitalization with risk of life-threatening toxicities, frequent in hospital visits and prolonged follow-up which heavily influence patients' quality of life. In this review we propose an innovative, telehealth-based, model for monitoring CAR-T patients: this method was used for managing a case of COVID-19 infection occurred two weeks after CAR-T cell infusion.
Methods: Several benefits for management of all these aspects of CAR-T programs could be made using telemedicine: for example, telemedicine real-time clinical monitoring could reduce the COVID-19 contagion risks for CAR-T patients.
Results: Our experience confirmed feasibility and utility of this approach in a real-life case. We believe that use of telemedicine for CAR-T patients could improve: the logistics of toxicity monitoring (frequent vital sign checks and neurologic assessments), the multidisciplinary team communication (patient selection, specialists consulting, coordination with pharmacists, etc.), the decrease in hospitalization time and the reduction of ambulatory visits.
Conclusions: This approach will be fundamental for future CAR-T cell program development, enhancing patients' quality of life and cost-effectiveness for healthcare systems.
Keywords: CAR-T; COVID-19; Cell therapy; Health system; Telehealth; Telemedicine.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Martino M, Naso V, Loteta B, Canale FA, Pugliese M, Alati C, Musuraca G, Nappi D, Gaimari A, Nicolini F, Mazza M, Bravaccini S, Derudas D, Martinelli G, Cerchione C. Chimeric antigen receptor T-cell therapy: what we expect soon. Int J Mol Sci. 2022;23(21):13332. doi: 10.3390/ijms232113332. - DOI - PMC - PubMed
-
- Yakoub-Agha I, Chabannon C, Bader P, et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2020;105(2):297–316. doi: 10.3324/haematol.2019.229781. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical