Effect of P2Y12 Inhibitors on Organ Support-Free Survival in Critically Ill Patients Hospitalized for COVID-19: A Randomized Clinical Trial
- PMID: 37227729
- PMCID: PMC10214036
- DOI: 10.1001/jamanetworkopen.2023.14428
Effect of P2Y12 Inhibitors on Organ Support-Free Survival in Critically Ill Patients Hospitalized for COVID-19: A Randomized Clinical Trial
Abstract
Importance: Platelet activation is a potential therapeutic target in patients with COVID-19.
Objective: To evaluate the effect of P2Y12 inhibition among critically ill patients hospitalized for COVID-19.
Design, setting, and participants: This international, open-label, adaptive platform, 1:1 randomized clinical trial included critically ill (requiring intensive care-level support) patients hospitalized with COVID-19. Patients were enrolled between February 26, 2021, through June 22, 2022. Enrollment was discontinued on June 22, 2022, by the trial leadership in coordination with the study sponsor given a marked slowing of the enrollment rate of critically ill patients.
Intervention: Participants were randomly assigned to receive a P2Y12 inhibitor or no P2Y12 inhibitor (usual care) for 14 days or until hospital discharge, whichever was sooner. Ticagrelor was the preferred P2Y12 inhibitor.
Main outcomes and measures: The primary outcome was organ support-free days, evaluated on an ordinal scale that combined in-hospital death and, for participants who survived to hospital discharge, the number of days free of cardiovascular or respiratory organ support up to day 21 of the index hospitalization. The primary safety outcome was major bleeding, as defined by the International Society on Thrombosis and Hemostasis.
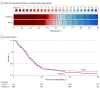
Results: At the time of trial termination, 949 participants (median [IQR] age, 56 [46-65] years; 603 male [63.5%]) had been randomly assigned, 479 to the P2Y12 inhibitor group and 470 to usual care. In the P2Y12 inhibitor group, ticagrelor was used in 372 participants (78.8%) and clopidogrel in 100 participants (21.2%). The estimated adjusted odds ratio (AOR) for the effect of P2Y12 inhibitor on organ support-free days was 1.07 (95% credible interval, 0.85-1.33). The posterior probability of superiority (defined as an OR > 1.0) was 72.9%. Overall, 354 participants (74.5%) in the P2Y12 inhibitor group and 339 participants (72.4%) in the usual care group survived to hospital discharge (median AOR, 1.15; 95% credible interval, 0.84-1.55; posterior probability of superiority, 80.8%). Major bleeding occurred in 13 participants (2.7%) in the P2Y12 inhibitor group and 13 (2.8%) in the usual care group. The estimated mortality rate at 90 days for the P2Y12 inhibitor group was 25.5% and for the usual care group was 27.0% (adjusted hazard ratio, 0.96; 95% CI, 0.76-1.23; P = .77).
Conclusions and relevance: In this randomized clinical trial of critically ill participants hospitalized for COVID-19, treatment with a P2Y12 inhibitor did not improve the number of days alive and free of cardiovascular or respiratory organ support. The use of the P2Y12 inhibitor did not increase major bleeding compared with usual care. These data do not support routine use of a P2Y12 inhibitor in critically ill patients hospitalized for COVID-19.
Trial registration: ClinicalTrials.gov Identifier: NCT04505774.
Conflict of interest statement
Figures