Identification of Hif1α as a Potential Participant in Autoimmune Uveitis Pathogenesis Using Single-Cell Transcriptome Analysis
- PMID: 37227746
- PMCID: PMC10214869
- DOI: 10.1167/iovs.64.5.24
Identification of Hif1α as a Potential Participant in Autoimmune Uveitis Pathogenesis Using Single-Cell Transcriptome Analysis
Abstract
Purpose: This study purposed to depict the transcriptional changes associated with autoimmune uveitis (AU) pathogenesis and identify potential therapeutic targets of this disease.
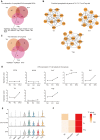
Methods: An experimental AU (EAU) model was established with retina antigen and adjuvants. An EAU control group was established with adjuvant only to eliminate nonspecific effects. We conducted single-cell RNA sequencing (scRNA-seq) on cervical draining lymph node cells of EAU, EAU control, and normal mice to identify the EAU-associated transcriptional changes and the potential pathogenic molecules. Subsequent flow cytometry, adoptive transfer experiment, scRNA-seq analysis of human uveitis, and proliferation assessment were conducted to verify the function of the interested molecule in uveitis.
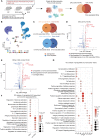
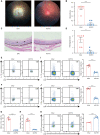
Results: The scRNA-seq data suggested that hypoxia-inducible factor 1 alpha (Hif1α) may participate in EAU pathogenesis via regulating T helper (Th)-17, Th1, and regulatory T cells. Hif1α inhibition alleviated EAU symptoms and regulated Th17, Th1, and regulatory T cell proportions. CD4+ T cells with repressed Hif1α expression failed to transfer EAU to naïve mice. In Vogt-Koyanagi-Harada disease, which is a human uveitis, Hif1α was also increased in CD4+ T cells and regulated their proliferation.
Conclusions: The results indicate that Hif1α may participate in AU pathogenesis and are, thus, a potential therapeutic target.
Conflict of interest statement
Disclosure:
Figures



Similar articles
-
Single-cell RNA sequencing indicates AP-1 as a potential therapeutic target for autoimmune uveitis.Biochem Pharmacol. 2025 Jul;237:116945. doi: 10.1016/j.bcp.2025.116945. Epub 2025 Apr 12. Biochem Pharmacol. 2025. PMID: 40228638
-
STING Deficiency Promotes Th17-Like Tfh to Aggravate the Experimental Autoimmune Uveitis.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):8. doi: 10.1167/iovs.66.3.8. Invest Ophthalmol Vis Sci. 2025. PMID: 40042874 Free PMC article.
-
Beneficial mechanisms of dimethyl fumarate in autoimmune uveitis: insights from single-cell RNA sequencing.J Neuroinflammation. 2024 Apr 29;21(1):112. doi: 10.1186/s12974-024-03096-6. J Neuroinflammation. 2024. PMID: 38684986 Free PMC article.
-
[Intraocular inflammation and homeostasis of the eye].Nippon Ganka Gakkai Zasshi. 2009 Mar;113(3):344-77; discussion 378. Nippon Ganka Gakkai Zasshi. 2009. PMID: 19348183 Review. Japanese.
-
[Translational research with experimental autoimmune uveoretinitis (EAU)].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):137-58; discussion 159. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402560 Review. Japanese.
Cited by
-
Eugenol ameliorates uveitis in mice with experimental autoimmune encephalomyelitis through the suppression of key inflammatory genes.Anim Cells Syst (Seoul). 2024 Jan 18;28(1):37-44. doi: 10.1080/19768354.2024.2304557. eCollection 2024. Anim Cells Syst (Seoul). 2024. PMID: 38249123 Free PMC article.
-
Baicalin prevents experimental autoimmune uveitis by promoting macrophage polarization balance through inhibiting the HIF-1α signaling pathway.Sci Rep. 2025 May 12;15(1):16424. doi: 10.1038/s41598-025-01286-7. Sci Rep. 2025. PMID: 40355594 Free PMC article.
References
-
- Krishna U, Ajanaku D, Denniston AK, Gkika T. Uveitis: a sight-threatening disease which can impact all systems. Postgrad Med J. 2017; 93(1106): 766–773. - PubMed
-
- Diedrichs-Möhring M, Kaufmann U ,Wildner G.. The immunopathogenesis of chronic and relapsing autoimmune uveitis - lessons from experimental rat models. Prog Retin Eye Res. 2018; 65: 107–126. - PubMed
-
- Burkholder BM,Jabs DA.. Uveitis for the non-ophthalmologist. BMJ (Clin Res Ed). 2021; 372(m4979. - PubMed
-
- Uchiyama E, Papaliodis GN, Lobo AM, Sobrin L. Side-effects of anti-inflammatory therapy in uveitis. Semin Ophthalmol. 2014; 29(5-6): 456–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

