The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection
- PMID: 37227774
- PMCID: PMC10371351
- DOI: 10.1172/jci.insight.167329
The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection
Abstract
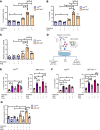
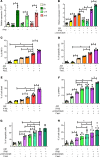
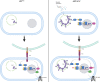
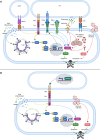
HIV-1 infection is characterized by inflammation and a progressive decline in CD4+ T cell count. Despite treatment with antiretroviral therapy (ART), the majority of people living with HIV (PLWH) maintain residual levels of inflammation, a low degree of immune activation, and higher sensitivity to cell death in their memory CD4+ T cell compartment. To date, the mechanisms responsible for this high sensitivity remain elusive. We have identified the transcription factor IRF-5 to be involved in impairing the maintenance of murine CD4+ T cells during chronic infection. Here, we investigate whether IRF-5 also contributes to memory CD4+ T cell loss during HIV-1 infection. We show that TLR7 and IRF-5 were upregulated in memory CD4+ T cells from PLWH, when compared with naturally protected elite controllers and HIVfree participants. TLR7 was upstream of IRF-5, promoting Caspase 8 expression in CD4+ T cells from ART HIV-1+ but not from HIVfree donors. Interestingly, the TLR7/IRF-5 axis acted synergistically with the Fas/FasL pathway, suggesting that TLR7 and IRF-5 expression in ART HIV-1+ memory CD4+ T cells represents an imprint that predisposes cells to Fas-mediated apoptosis. This predisposition could be blocked using IRF-5 inhibitory peptides, suggesting IRF-5 blockade as a possible therapy to prevent memory CD4+ T cell loss in PLWH.
Keywords: AIDS/HIV; Apoptosis; Immunology; T cells.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

