Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice
- PMID: 37227777
- PMCID: PMC10313368
- DOI: 10.1172/JCI163290
Targeting hypoxia-inducible factors with 32-134D safely and effectively treats diabetic eye disease in mice
Abstract
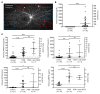
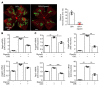
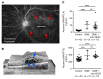
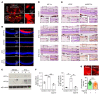
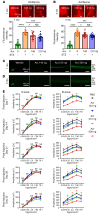
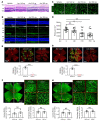
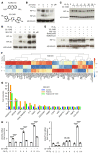
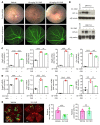
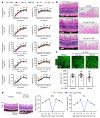
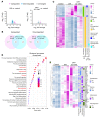
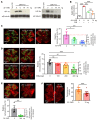
Many patients with diabetic eye disease respond inadequately to anti-VEGF therapies, implicating additional vasoactive mediators in its pathogenesis. We demonstrate that levels of angiogenic proteins regulated by HIF-1 and -2 remain elevated in the eyes of people with diabetes despite treatment with anti-VEGF therapy. Conversely, by inhibiting HIFs, we normalized the expression of multiple vasoactive mediators in mouse models of diabetic eye disease. Accumulation of HIFs and HIF-regulated vasoactive mediators in hyperglycemic animals was observed in the absence of tissue hypoxia, suggesting that targeting HIFs may be an effective early treatment for diabetic retinopathy. However, while the HIF inhibitor acriflavine prevented retinal vascular hyperpermeability in diabetic mice for several months following a single intraocular injection, accumulation of acriflavine in the retina resulted in retinal toxicity over time, raising concerns for its use in patients. Conversely, 32-134D, a recently developed HIF inhibitor structurally unrelated to acriflavine, was not toxic to the retina, yet effectively inhibited HIF accumulation and normalized HIF-regulated gene expression in mice and in human retinal organoids. Intraocular administration of 32-134D prevented retinal neovascularization and vascular hyperpermeability in mice. These results provide the foundation for clinical studies assessing 32-134D for the treatment of patients with diabetic eye disease.
Keywords: Diabetes; Mouse models; Ophthalmology; Retinopathy; Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

