Characterization of industry relationships in oncology
- PMID: 37227811
- PMCID: PMC12036008
- DOI: 10.1002/cncr.34852
Characterization of industry relationships in oncology
Abstract
Background: Collaborative relationships between academic oncology and industry (pharmaceutical, biotechnology, "omic," and medical device companies) are essential for therapeutic development in oncology; however, limited research on engagement in and perceptions of these relationships has been done.
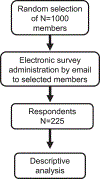
Methods: Survey questions were developed to evaluate relationships between academic oncology and industry. An electronic survey was delivered to 1000 randomly selected members of the American Society of Clinical Oncology, a professional organization for oncologists, eliciting respondents' views around oncology-industry collaborations. The responses were analyzed according to prespecified plans.
Results: There were 225 survey respondents. Most were from the United States (70.0%), worked at an academic institution (60.1%), worked in medical oncology (81.2%), and had an active relationship with industry (85.8%). One quarter (26.7%) of respondents reported difficulty establishing a relationship with industry collaborators, and most respondents (75%) did not report having had mentorship in developing these relationships. The majority (85.3%) of respondents considered these collaborations important to their careers. Respondents generally thought that scientific integrity was preserved (92%), and most respondents (95%) had little concern over the quality of the collaborative product. Many (60%) shared concerns over potential conflict of interest if an individual with a compensated relationship promoted an industry product for clinical care/research, yet most respondents (67%) stated these relationships did not shape their interactions with patients.
Conclusions: This study provides novel data characterizing the nature of collaborative relationships between clinicians, researchers, and industry in oncology. Although respondents considered these collaborations an important part of clinical and academic oncology, formal education or mentorship around these relationships was rare. Conflicting findings around conflict of interest highlight the importance of more dedicated research in this area.
Plain language summary: Business enterprises in health care play a central role in cancer research and care, driving the development of new medical testing, drugs, and devices. Effective working relationships among clinicians, researchers, and these industry partners can promote innovative research and enhance patient care. Study of these collaborations has been limited to date. Through distribution of a questionnaire to cancer clinicians and researchers, we found that most participants consider these relationships valuable, though they find establishing such relationships challenging partly because of gaps in educational programs in this area. Our findings also highlight the need for further policy around the potential bias these relationships can introduce.
Keywords: clinical trials; collaboration; conflicts of interest; pharmaceutical industry; protocol development.
© 2023 American Cancer Society.
Conflict of interest statement
The majority of respondents (59.9%) supported the concern that a compensated relationship constitutes a COI if the specific industry products are promoted in clinical research or patient care, while over half (67.6%) shared that their relationships with industry did not shape their own proposals, therapies, or discussions with patients. When asked why, respondents selected having an institutional formal conflict management policy (23.9%), having collaborations with many industry partners so that if there is a conflict it will be equalized among them, and not allowing these relationships to influence decisions (49.3%) as reasons for navigating these relationships. Most respondents shared that their institutions had a formal COI-reporting policy (81.8%), with most requiring annual COI reporting (68.8%) as opposed to per-contact reporting (28.5%) or other reporting methods (2.8%). About one quarter (26.6%) reported their institutions had optional reporting guidelines.
Thirty-nine percent of respondents felt their institutions did not provide sufficient guidance around COI in industry relationships and that current standards and guidance on COI in these relationships are inadequate (39.5%). Notably, most respondents did not share a significant concern that their industry relationships had significant influence over the clinical care they provided (Figure 4).
Figures


References
-
- Riechelmann RP, et al., Disclosure of Conflicts of Interest by Authors of Clinical Trials and Editorials in Oncology. Journal of Clinical Oncology, 2007. 25(29): p. 4642–4647. - PubMed
-
- Moses H 3rd, et al., The anatomy of medical research: US and international comparisons. Jama, 2015. 313(2): p. 174–89. - PubMed
-
- Wright K, et al., Industry Relationships With Medical Oncologists: Who Are the High-Payment Physicians? JCO Oncol Pract, 2022. 18(7): p. e1164–e1169. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

