Randomized Trial to Assess the Clinical Utility of Renal Allograft Monitoring by Urine CXCL10 Chemokine
- PMID: 37228005
- PMCID: PMC10400101
- DOI: 10.1681/ASN.0000000000000160
Randomized Trial to Assess the Clinical Utility of Renal Allograft Monitoring by Urine CXCL10 Chemokine
Abstract
Significance statement: This study is the first randomized controlled trial to investigate the clinical utility of a noninvasive monitoring biomarker in renal transplantation. Although urine CXCL10 monitoring could not demonstrate a beneficial effect on 1-year outcomes, the study is a rich source for future design of trials aiming to explore the clinical utility of noninvasive biomarkers. In addition, the study supports the use of urine CXCL10 to assess the inflammatory status of the renal allograft.
Background: Urine CXCL10 is a promising noninvasive biomarker for detection of renal allograft rejection. The aim of this study was to investigate the clinical utility of renal allograft monitoring by urine CXCL10 in a randomized trial.
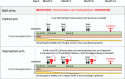
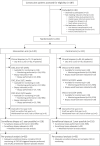
Methods: We stratified 241 patients, 120 into an intervention and 121 into a control arm. In both arms, urine CXCL10 levels were monitored at three specific time points (1, 3, and 6 months post-transplant). In the intervention arm, elevated values triggered performance of an allograft biopsy with therapeutic adaptations according to the result. In the control arm, urine CXCL10 was measured, but the results concealed. The primary outcome was a combined end point at 1-year post-transplant (death-censored graft loss, clinical rejection between month 1 and 1-year, acute rejection in 1-year surveillance biopsy, chronic active T-cell-mediated rejection in 1-year surveillance biopsy, development of de novo donor-specific HLA antibodies, or eGFR <25 ml/min).
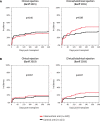
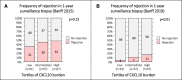
Results: The incidence of the primary outcome was not different between the intervention and the control arm (51% versus 49%; relative risk (RR), 1.04 [95% confidence interval, 0.81 to 1.34]; P = 0.80). When including 175 of 241 (73%) patients in a per-protocol analysis, the incidence of the primary outcome was also not different (55% versus 49%; RR, 1.11 [95% confidence interval, 0.84 to 1.47]; P = 0.54). The incidence of the individual end points was not different as well.
Conclusions: This study could not demonstrate a beneficial effect of urine CXCL10 monitoring on 1-year outcomes (ClinicalTrials.gov_ NCT03140514 ).
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
All authors have nothing to disclose.
Figures


Comment in
-
Of End Points and Context of Use: A Reasonable Silver Lining for Urinary Chemokines Monitoring.J Am Soc Nephrol. 2023 Oct 1;34(10):1765-1766. doi: 10.1681/ASN.0000000000000206. Epub 2023 Aug 18. J Am Soc Nephrol. 2023. PMID: 37782546 Free PMC article. No abstract available.
-
Authors' Reply: Of End Points and Context of Use: A Reasonable Silver Lining for Urinary Chemokines Monitoring.J Am Soc Nephrol. 2023 Oct 1;34(10):1766-1767. doi: 10.1681/ASN.0000000000000205. Epub 2023 Aug 18. J Am Soc Nephrol. 2023. PMID: 37782547 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

