EPCR deficiency ameliorates inflammatory arthritis in mice by suppressing the activation and migration of T cells and dendritic cells
- PMID: 37228024
- PMCID: PMC10834933
- DOI: 10.1093/rheumatology/kead230
EPCR deficiency ameliorates inflammatory arthritis in mice by suppressing the activation and migration of T cells and dendritic cells
Abstract
Objectives: Endothelial protein C receptor (EPCR) is highly expressed in synovial tissues of patients with RA, but the function of this receptor remains unknown in RA. This study investigated the effect of EPCR on the onset and development of inflammatory arthritis and its underlying mechanisms.
Methods: CIA was induced in EPCR gene knockout (KO) and matched wild-type (WT) mice. The onset and development of arthritis was monitored clinically and histologically. T cells, dendritic cells (DCs), EPCR and cytokines from EPCR KO and WT mice, RA patients and healthy controls (HCs) were detected by flow cytometry and ELISA.
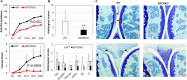
Results: EPCR KO mice displayed >40% lower arthritis incidence and 50% less disease severity than WT mice. EPCR KO mice also had significantly fewer Th1/Th17 cells in synovial tissues with more DCs in circulation. Lymph nodes and synovial CD4 T cells from EPCR KO mice expressed fewer chemokine receptors CXCR3, CXCR5 and CCR6 than WT mice. In vitro, EPCR KO spleen cells contained fewer Th1 and more Th2 and Th17 cells than WT and, in concordance, blocking EPCR in WT cells stimulated Th2 and Th17 cells. DCs generated from EPCR KO bone marrow were less mature and produced less MMP-9. Circulating T cells from RA patients expressed higher levels of EPCR than HC cells; blocking EPCR stimulated Th2 and Treg cells in vitro.
Conclusion: Deficiency of EPCR ameliorates arthritis in CIA via inhibition of the activation and migration of pathogenic Th cells and DCs. Targeting EPCR may constitute a novel strategy for future RA treatment.
Keywords: CIA; RA; Th cells; cytokines; dendritic cells; endothelial protein C receptor.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures





Similar articles
-
Endothelial Protein C Receptor: A Multifunctional Mediator in the Pathophysiology of Rheumatoid Arthritis.Cells. 2025 Mar 24;14(7):485. doi: 10.3390/cells14070485. Cells. 2025. PMID: 40214439 Free PMC article. Review.
-
Elevated levels of soluble Endothelial protein C receptor in rheumatoid arthritis and block the therapeutic effect of protein C in collagen-induced arthritis.Int Immunopharmacol. 2020 Apr;81:106255. doi: 10.1016/j.intimp.2020.106255. Epub 2020 Jan 30. Int Immunopharmacol. 2020. PMID: 32007797
-
Deficiency of protease-activated receptor (PAR) 1 and PAR2 exacerbates collagen-induced arthritis in mice via differing mechanisms.Rheumatology (Oxford). 2021 Jun 18;60(6):2990-3003. doi: 10.1093/rheumatology/keaa701. Rheumatology (Oxford). 2021. PMID: 33823532
-
Myeloid deletion of SIRT1 suppresses collagen-induced arthritis in mice by modulating dendritic cell maturation.Exp Mol Med. 2016 Mar 18;48(3):e221. doi: 10.1038/emm.2015.124. Exp Mol Med. 2016. PMID: 26987484 Free PMC article.
-
Blocking the Sphingosine-1-Phosphate Receptor 2 (S1P2) Reduces the Severity of Collagen-Induced Arthritis in DBA-1J Mice.Int J Mol Sci. 2024 Dec 13;25(24):13393. doi: 10.3390/ijms252413393. Int J Mol Sci. 2024. PMID: 39769163 Free PMC article.
Cited by
-
Exploring the association between circulating endothelial protein C receptor and disease activity of rheumatoid arthritis in a pilot study.Rheumatol Adv Pract. 2024 Aug 6;8(3):rkae096. doi: 10.1093/rap/rkae096. eCollection 2024. Rheumatol Adv Pract. 2024. PMID: 39184533 Free PMC article.
-
Optineurin restrains CCR7 degradation to guide type II collagen-stimulated dendritic cell migration in rheumatoid arthritis.Acta Pharm Sin B. 2025 Mar;15(3):1626-1642. doi: 10.1016/j.apsb.2025.02.004. Epub 2025 Feb 11. Acta Pharm Sin B. 2025. PMID: 40370566 Free PMC article.
-
Endothelial Protein C Receptor and Its Impact on Rheumatic Disease.J Clin Med. 2024 Mar 31;13(7):2030. doi: 10.3390/jcm13072030. J Clin Med. 2024. PMID: 38610795 Free PMC article. Review.
-
Endothelial Protein C Receptor: A Multifunctional Mediator in the Pathophysiology of Rheumatoid Arthritis.Cells. 2025 Mar 24;14(7):485. doi: 10.3390/cells14070485. Cells. 2025. PMID: 40214439 Free PMC article. Review.
References
-
- O’Neil LJ, Kaplan MJ.. Neutrophils in rheumatoid arthritis: breaking immune tolerance and fueling disease. Trends Mol Med 2019;25:215–27. - PubMed
-
- Yang P, Qian FY, Zhang MF. et al. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J Leukoc Biol 2019;106:1233–40. - PubMed
-
- Gizinski AM, Fox DA.. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol 2014;26:204–10. - PubMed
-
- Esmon CT. Structure and functions of the endothelial cell protein C receptor. Crit Care Med 2004;32(5 Suppl):S298–S301. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

