Sodium Bicarbonate Treatment and Vascular Function in CKD: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 37228030
- PMCID: PMC10400105
- DOI: 10.1681/ASN.0000000000000161
Sodium Bicarbonate Treatment and Vascular Function in CKD: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Significance statement: Lower serum bicarbonate levels, even within the normal range, are strongly linked to risks of cardiovascular disease in CKD, possibly by modifying vascular function. In this randomized, controlled trial, treatment with sodium bicarbonate (NaHCO 3 ) did not improve vascular endothelial function or reduce arterial stiffness in participants with CKD stage 3b-4 with normal serum bicarbonate levels. In addition, NaHCO 3 treatment did not reduce left ventricular mass index. NaHCO 3 did increase plasma bicarbonate levels and urinary citrate excretion and reduce urinary ammonium excretion, indicating that the intervention was indeed effective. NaHCO 3 therapy was safe with no significant changes in BP, weight, or edema. These results do not support the use of NaHCO 3 for vascular dysfunction in participants with CKD.
Background: Lower serum bicarbonate levels, even within the normal range, are strongly linked to risks of cardiovascular disease in CKD, possibly by modifying vascular function. Prospective interventional trials with sodium bicarbonate (NaHCO 3 ) are lacking.
Methods: We conducted a randomized, double-blind, placebo-controlled trial examining the effect of NaHCO 3 on vascular function in 109 patients with CKD stage 3b-4 (eGFR 15-44 ml/min per 1.73 m 2 ) with normal serum bicarbonate levels (22-27 mEq/L). Participants were randomized 1:1 to NaHCO 3 or placebo at a dose of 0.5 mEq/lean body weight-kg per day for 12 months. The coprimary end points were change in brachial artery flow-mediated dilation (FMD) and change in aortic pulse wave velocity over 12 months.
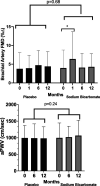
Results: Ninety patients completed this study. After 12 months, plasma bicarbonate levels increased significantly in the NaHCO 3 group compared with placebo (mean [SD] difference between groups 1.35±2.1, P = 0.003). NaHCO 3 treatment did not result in a significant improvement in aortic pulse wave velocity from baseline. NaHCO 3 did result in a significant increase in flow-mediated dilation after 1 month; however, this effect disappeared at 6 and 12 months. NaHCO 3 resulted in a significant increase in 24-hour urine citrate and pH and a significant decrease in 24-hour urine ammonia. There was no significant change in left ventricular mass index, ejection fraction, or eGFR with NaHCO 3 . NaHCO 3 treatment was safe and well-tolerated with no significant changes in BP, antihypertensive medication, weight, plasma calcium, or potassium levels.
Conclusion: Our results do not support the use of NaHCO 3 for vascular dysfunction in participants with CKD and normal serum bicarbonate levels.
Trial registration: ClinicalTrials.gov NCT02915601.
Copyright © 2023 by the American Society of Nephrology.
Conflict of interest statement
J. Kendrick has received advisory fees from AstraZeneca. J. Kendrick has received grant funding from AstraZeneca, Bayer Healthcare, Fresenius Renal Therapies, NHBLI, NIA, NIH NIDDK, and the Juvenile Diabetes Research Foundation. K.L. Nowak has received consulting fees from Otsuka and honoraria from the National Kidney Foundation. K.L. Nowak has received grant funding from the NIH and Polycystic Kidney Disease (PKD) Foundation. M. Chonchol has received grant funding from NIH. All remaining authors have nothing to disclose.
Figures


Comment in
-
Does Acid Stress Cause Vascular Dysfunction?J Am Soc Nephrol. 2023 Aug 1;34(8):1299-1301. doi: 10.1681/ASN.0000000000000162. J Am Soc Nephrol. 2023. PMID: 37526983 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

