Tazemetostat for tumors harboring SMARCB1/SMARCA4 or EZH2 alterations: results from NCI-COG pediatric MATCH APEC1621C
- PMID: 37228094
- PMCID: PMC11009504
- DOI: 10.1093/jnci/djad085
Tazemetostat for tumors harboring SMARCB1/SMARCA4 or EZH2 alterations: results from NCI-COG pediatric MATCH APEC1621C
Abstract
Background: National Cancer Institute-Children's Oncology Group Pediatric Molecular Analysis for Therapy Choice assigns patients aged 1-21 years with refractory solid tumors, brain tumors, lymphomas, and histiocytic disorders to phase II trials of molecularly targeted therapies based on detection of predefined genetic alterations. Patients whose tumors harbored EZH2 mutations or loss of SMARCB1 or SMARCA4 by immunohistochemistry were treated with EZH2 inhibitor tazemetostat.
Methods: Patients received tazemetostat for 28-day cycles until disease progression or intolerable toxicity (max 26 cycles). The primary endpoint was objective response rate; secondary endpoints included progression-free survival and tolerability of tazemetostat.
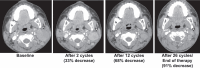
Results: Twenty patients (median age = 5 years) enrolled, all evaluable for response and toxicities. The most frequent diagnoses were atypical teratoid rhabdoid tumor (n = 8) and malignant rhabdoid tumor (n = 4). Actionable alterations consisted of SMARCB1 loss (n = 16), EZH2 mutation (n = 3), and SMARCA4 loss (n = 1). One objective response was observed in a patient with non-Langerhans cell histiocytosis with SMARCA4 loss (26 cycles, 1200 mg/m2/dose twice daily). Four patients with SMARCB1 loss had a best response of stable disease: epithelioid sarcoma (n = 2), atypical teratoid rhabdoid tumor (n = 1), and renal medullary carcinoma (n = 1). Six-month progression-free survival was 35% (95% confidence interval [CI] = 15.7% to 55.2%) and 6-month overall survival was 45% (95% CI = 23.1% to 64.7%). Treatment-related adverse events were consistent with prior tazemetostat reports.
Conclusions: Although tazemetostat did not meet its primary efficacy endpoint in this population of refractory pediatric tumors (objective response rate = 5%, 90% CI = 1% to 20%), 25% of patients with multiple histologic diagnoses experienced prolonged stable disease of 6 months and over (range = 9-26 cycles), suggesting a potential effect of tazemetostat on disease stabilization.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Susan Chi: Consulting or advisory role—Epizyme; Blueprint Medicines. Stacey L. Berg: travel, accommodations, expenses—nonprofit. Other relationship—member of the COG Developmental Therapeutics Steering Committee, through which some clinical trials may be partially industry funded, institution may receive some funding, Pediatric Early Phase Clinical Trials Network. Elizabeth Fox: other relationship—Helsinn Therapeutics. Douglas S. Hawkins: research funding—Amgen; Bayer (Inst); Bristol-Myers Squibb (Inst); Eisai (Inst); Incyte; Jazz Pharmaceuticals; Lilly (Inst); Loxo (Inst); Merck Sharp & Dohme (Inst); Seattle Genetics. Katherine A. Janeway: honoraria—Foundation Medicine and Takeda. Consulting or advisory role—Bayer; Ipsen. Travel, accommodations, expenses—Bayer. D. Williams Parsons: Patents, royalties, other intellectual property—Co-inventor on current and pending patents related to cancer genes discovered through sequencing of several adult cancer types. Participates in royalty sharing related to those patents.
Elizabeth Fox, a
Figures



Comment in
-
EZH2 inhibition: it's all about the context.J Natl Cancer Inst. 2023 Nov 8;115(11):1246-1248. doi: 10.1093/jnci/djad141. J Natl Cancer Inst. 2023. PMID: 37682251 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

