Identification of upper thermal thresholds during development in the endangered Nechako white sturgeon with management implications for a regulated river
- PMID: 37228298
- PMCID: PMC10205467
- DOI: 10.1093/conphys/coad032
Identification of upper thermal thresholds during development in the endangered Nechako white sturgeon with management implications for a regulated river
Abstract
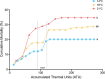
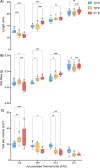
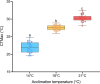
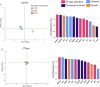
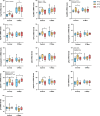
Climate change-induced warming effects are already evident in river ecosystems, and projected increases in temperature will continue to amplify stress on fish communities. In addition, many rivers globally are impacted by dams, which have many negative effects on fishes by altering flow, blocking fish passage, and changing sediment composition. However, in some systems, dams present an opportunity to manage river temperature through regulated releases of cooler water. For example, there is a government mandate for Kenney dam operators in the Nechako river, British Columbia, Canada, to maintain river temperature <20°C in July and August to protect migrating sockeye salmon (Oncorhynchus nerka). However, there is another endangered fish species inhabiting the same river, Nechako white sturgeon (Acipenser transmontanus), and it is unclear if these current temperature regulations, or timing of the regulations, are suitable for spawning and developing sturgeon. In this study, we aimed to identify upper thermal thresholds in white sturgeon embryos and larvae to investigate if exposure to current river temperatures are playing a role in recruitment failure. We incubated embryos and yolk-sac larvae in three environmentally relevant temperatures (14, 18 and 21°C) throughout development to identify thermal thresholds across different levels of biological organization. Our results demonstrate upper thermal thresholds at 21°C across physiological measurements in embryo and yolk-sac larvae white sturgeon. Before hatch, both embryo survival and metabolic rate were reduced at 21°C. After hatch, sublethal consequences continued at 21°C because larval sturgeon had decreased thermal plasticity and a dampened transcriptional response during development. In recent years, the Nechako river has reached 21°C by the end of June, and at this temperature, a decrease in sturgeon performance is evident in most of the traits measured. As such, the thermal thresholds identified here suggest current temperature regulations may not be suitable for developing white sturgeon and future recruitment.
Keywords: Conservation; development; sturgeon.
© The Author(s) 2023. Published by Oxford University Press and the Society for Experimental Biology.
Figures


References
-
- Allen PJ, Nicholl M, Cole S, Vlazny A, Cech JJ (2006) Growth of larval to juvenile Green sturgeon in elevated temperature regimes. Trans Am Fish Soc 135: 89–96. 10.1577/T05-020.1. - DOI
-
- Anderson JJ, Beer WN, Israel JA, Greene S (2022) Targeting river operations to the critical thermal window of fish incubation: model and case study on Sacramento River winter-run Chinook salmon. River Research & Apps 38: 895–905. 10.1002/rra.3965. - DOI
-
- Baker DW, McAdam DSO, Boucher M, Huynh KT, Brauner CJ (2014) Swimming performance and larval quality are altered by rearing substrate at early life phases in white sturgeon, Acipenser transmontanus (Richardson, 1836). J Appl Ichthyol 30: 1461–1472. 10.1111/jai.12642. - DOI
-
- Bard B, Kieffer JD (2019) The effects of repeat acute thermal stress on the critical thermal maximum (CTmax) and physiology of juvenile shortnose sturgeon (Acipenser brevirostrum). Can J Zool 97: 567–572. 10.1139/cjz-2018-0157. - DOI
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

