Benzo[ g]quinazolines as antifungal against candidiasis: Screening, molecular docking, and QSAR investigations
- PMID: 37228321
- PMCID: PMC10203769
- DOI: 10.1016/j.jsps.2023.04.012
Benzo[ g]quinazolines as antifungal against candidiasis: Screening, molecular docking, and QSAR investigations
Abstract
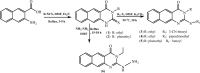
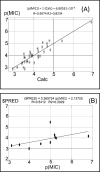
Candida albicans, an opportunistic pathogen, is the most common type of fungus and represents a substantial source of human invasive disease (nosocomial infection). This category of fungi are part of our microbiota, and given the appropriate environmental conditions, it has the potential to cause both superficial and systemic infections. There is a soaring resistance against the available anticandidal agents. The purpose of this research is to investigate the activity of certain previously synthesized benzo[g]quinazolines against C. albicans in vitro by using the cup-plate diffusion method. There was a marked difference in the effectiveness of the target compounds 1-6 against the sample of C. albicans that was tested. Benzo[g]quinazolines 1 (inhibition zone = 20 mm) and 2 (inhibition zone = 22 mm) had good effects in comparison to fluconazole (inhibition zone = 26 mm). A docking study was conducted between benzo[g]quinazolines 1-6 and Candida spp. CYP51 to establish the binding mode compared with fluconazole and VT-1161 (oteseconazole) as reference medicines, and it was determined that binding at the active site of Candida spp. CYP51 occurred in the same manner. Quantitative structure-activity relationship (QSAR) investigation was performed to further characterize the identified anticandidal agents and recognize the major regulatory components governing such activity. In future studies, the benzo[g]quinazoline scaffold could serve as a model for the design and development of novel derivatives with antifungal potential.
Keywords: Anticandidal agent; Benzo[g]quinazolines; CYP51; Candida albicans; Molecular docking; Quantitative structure–activity relationship.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Discovery of new azoles with potent activity against Candida spp. and Candida albicans biofilms through virtual screening.Eur J Med Chem. 2019 Oct 1;179:634-648. doi: 10.1016/j.ejmech.2019.06.083. Epub 2019 Jun 29. Eur J Med Chem. 2019. PMID: 31279296
-
Antifungal Activity, Gas Chromatographic-Mass Spectrometric Analysis And in silico Study of Punica Granatum Peel Extracts Against Fluconazole Resistant Strains of Candida Species.Curr Pharm Biotechnol. 2018;19(3):250-257. doi: 10.2174/1389201019666180515104800. Curr Pharm Biotechnol. 2018. PMID: 29766800
-
In vivo and in vitro activity of a bis-arylidenecyclo-alkanone against fluconazole-susceptible and -resistant isolates of Candida albicans.J Glob Antimicrob Resist. 2018 Sep;14:287-293. doi: 10.1016/j.jgar.2018.04.012. Epub 2018 Apr 30. J Glob Antimicrob Resist. 2018. PMID: 29715565
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology.Drugs. 2009;69(3):339-59. doi: 10.2165/00003495-200969030-00009. Drugs. 2009. PMID: 19275277 Review.
Cited by
-
Design, synthesis, and carbonic anhydrase inhibition activities of Schiff bases incorporating benzenesulfonamide scaffold: Molecular docking application.Saudi Pharm J. 2023 Dec;31(12):101866. doi: 10.1016/j.jsps.2023.101866. Epub 2023 Nov 10. Saudi Pharm J. 2023. PMID: 38033749 Free PMC article.
-
Multifunctional in vitro, in silico and DFT analyses on antimicrobial BagremycinA biosynthesized by Micromonospora chokoriensis CR3 from Hieracium canadense.Sci Rep. 2024 May 14;14(1):10976. doi: 10.1038/s41598-024-61490-9. Sci Rep. 2024. PMID: 38745055 Free PMC article.
-
Molecular Modeling of Brassicaceae Derivatives for Inhibiting Lipoxygenases: A Promising Therapeutic Strategy.Curr Drug Discov Technol. 2024;21(4):48-e011223224117. doi: 10.2174/0115701638269042231122064738. Curr Drug Discov Technol. 2024. PMID: 39206704
-
Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review.Antibiotics (Basel). 2023 Jul 22;12(7):1220. doi: 10.3390/antibiotics12071220. Antibiotics (Basel). 2023. PMID: 37508316 Free PMC article. Review.
-
Chitosan-enclosed SLN improved SV-induced hepatocellular cell carcinoma death by modulation of IQGAP gene expression, JNK, and HDAC activities.Mol Biol Rep. 2024 Jul 18;51(1):824. doi: 10.1007/s11033-024-09757-2. Mol Biol Rep. 2024. PMID: 39023688
References
-
- Abuelizz H.A., El-Dib R.A., Marzouk M., Al-Salahi R. In vitro evaluation of new 2-phenoxy-benzo[g][1,2,4]triazolo[1,5-a]quinazoline derivatives as antimicrobial agents. Microb. Pathog. 2018;117:60–67. - PubMed
-
- Abuelizz H.A., Awad H.M., Marzouk M., Nasr F.A., Bakheit A.H., Naglah A.M., Al-Shakliah N.S., Al-Salahi R. Exploiting the 4-Hydrazinobenzoic acid moiety for the development of anticancer agents: Synthesis and biological profile. Bioorg. Chem. 2020;102 - PubMed
-
- Abuelizz H.A., Marzouk M., Bakhiet A., Abdel-Aziz M.M., Ezzeldin E., Rashid H., Al-Salahi R. In silico study and biological screening of benzoquinazolines as potential antimicrobial agents against methicillin-resistant Staphylococcus aureus, carbapenem-resistant Klebsiella pneumoniae, and fluconazole-resistant Candida albicans. Microb. Pathog. 2021;160 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

