Design, physicochemical characterisation, and in vitro cytotoxicity of cisplatin-loaded PEGylated chitosan injectable nano / sub-micron crystals
- PMID: 37228326
- PMCID: PMC10203781
- DOI: 10.1016/j.jsps.2023.04.005
Design, physicochemical characterisation, and in vitro cytotoxicity of cisplatin-loaded PEGylated chitosan injectable nano / sub-micron crystals
Abstract
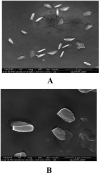
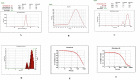
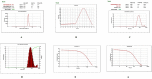
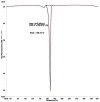
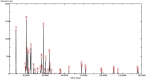
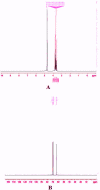
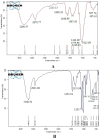
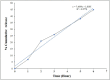
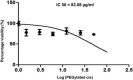
The study aimed to develop cisplatin-loaded PEGylated chitosan nanoparticles. The optimal batch of cisplatin-loaded PEGylated chitosan nanoparticles had a + 49.9 mV zeta potential, PDI of 0.347, and % PDI of 58.9. Nanoparticle zeta size was 741.4 z. d.nm, the size in diameter was 866.7 ± 470.5 nm, and nanoparticle conductivity in colloidal solution was 0.739 mS/cm. Differential scanning calorimetry (DSC) revealed that cisplatin-loaded PEGylated chitosan nanoparticles had sharp endothermic peaks at temperatures at 168.6 °C. The thermogravimetric analysis (TGA) showed the weight loss of cisplatin-loaded PEGylated chitosan nanoparticles, which was observed as 95% at 262.76 °C. XRD investigation on cisplatin-loaded PEGylated chitosan nanoparticles exhibited distinct peaks at 2θ as 9.7°, 20.4°, 22.1°, 25.3°, 36.1°, 38.1°, 39.5°, 44.3°, and 64.5°, confirming crystalline structure. The 1H NMR analysis showed the fingerprint region of cisplatin-loaded PEGylated chitosan nanoparticles as 0.85, 1.73, and 1.00 ppm in the proton dimension and de-shielded proton peaks appeared at 3.57, 3.58, 3.58, 3.59, 3.65, 3.67, 3,67, 3,67, 3.70, 3.71, 3.77, 3.78 and 4.71 ppm. The 13C NMR spectrum showed specified peaks at 63.18, 69.20, and 70.77 ppm. The FT-IR spectra of cisplatin loaded PEGylated nanoparticles show the existence of many fingerprint regions at 3186.52, 2931.68, 1453.19, 1333.98, 1253.71, 1085.19, 1019.60, 969.98, 929.53, 888.80, 706.13, and 623.67 cm-1. The drug release kinetics of cisplatin loaded PEGylated chitosan nanoparticles showed zero order kinetics with 48% of drug release linearity fashion which has R2 value of 0.9778. Studies on the MCF-7 ATCC human breast cancer cell line in vitro revealed that the IC50 value 82.08 µg /mL. Injectable nanoparticles had good physicochemical and cytotoxic properties. This method is novel since the application of the PEGylation processes leads to an increased solubility of chitosan nanoparticles at near neutral pH.
Keywords: Cancer; Cisplatin; Cytotoxicity; Nanoparticles; Pegylated Chitosan; Polydispersity Index; Size; Zetapotential.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Abouelhag H.A., Sivakumar S.M., Bagul U.S., Mohamed Eltyep E., Safhi M.M. Preparation and physical characterization of cisplatin-chitosan nanoparticles by zeta nanosizer “prime step for formulation and development”. Int. J. Pharm. Sci. Res. 2017;10:4245–4249. doi: 10.13040/IJPSR.0975-8232.8(10).4245-49. - DOI
-
- Ahmed A.M., Ali M.R., Noor A.K. Use of biocomposite adsorbents for the removal of methylene blue dye from aqueous solution. Am. J. Mater. Sci. 2016;6(5):135–146. doi: 10.5923/j.materials.20160605.03. - DOI
-
- Anand M., Sathyapriya P., Maruthupandy M., Beevi A.H. Synthesis of chitosan nanoparticles by TPP and their potential mosquito larvicidal application. Front. Lab. Med. 2018;2:72–78. doi: 10.1016/j.flm.2018.07.003. - DOI
LinkOut - more resources
Full Text Sources

