GADD34 activates p53 and may have utility as a marker of atherosclerosis
- PMID: 37228401
- PMCID: PMC10203227
- DOI: 10.3389/fmed.2023.1128921
GADD34 activates p53 and may have utility as a marker of atherosclerosis
Abstract
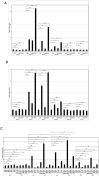
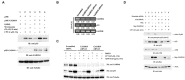
We previously identified growth arrest and DNA-damage-inducible gene 34 (GADD34) as a marker of ischemic stroke. In the present study, serum levels of anti-GADD34 antibodies were found to be significantly higher in patients with acute ischemic stroke or chronic kidney disease compared to healthy donors. We then examined the biological function of GADD34 by transfection into U2OS human osteosarcoma and U87 human glioblastoma cells. Knockdown of GADD34 by siRNA resulted in enhanced cell proliferation, which was reversed by co-knockdown of MDM2. Luciferase reporter assays revealed that the transactivation ability of p53 enhanced by genotoxic anticancer drugs such as camptothecin and etoposide was further potentiated by enforced expression of GADD34 but attenuated by co-transfection with p53 shRNA expression plasmids. Western blotting demonstrated increased p53 protein levels after treatment with camptothecin, which was also potentiated by GADD34 but suppressed by GADD34 siRNA, ATM siRNA, and ATM inhibitor wortmannin. GADD34 levels also increased in response to treatment with camptothecin or adriamycin, and this increase was attenuated by MDM2 siRNA. Immunoprecipitation with anti-GADD34 antibody followed by Western blotting with anti-MDM2 antibodies indicated ubiquitination of GADD34 is mediated by MDM2. Accordingly, GADD34 may function as a ubiquitination decoy to reduce p53 ubiquitination and increase p53 protein levels. Increased neuronal cell death due to activation of p53 by GADD34 may account for the elevated serum levels of anti-GADD34 antibodies observed in patients with acute ischemic stroke.
Keywords: GADD34; atherosclerosis; ischemic stroke; p53; ubiquitination.
Copyright © 2023 Tomiyoshi, Nakamura, Shinmen, Yoshida, Mine, Machida, Iwase, Iwadate, Hiwasa and Kuroda.
Conflict of interest statement
This work was performed in collaboration with Fujikura Kasei Co., Ltd. GT, RN, NS, and HK are employee of Fujikura Kasei Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. (1994) 54:4855–78. PMID: - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

