Plasma protein changes reflect colorectal cancer development and associated inflammation
- PMID: 37228491
- PMCID: PMC10203952
- DOI: 10.3389/fonc.2023.1158261
Plasma protein changes reflect colorectal cancer development and associated inflammation
Abstract
Introduction: Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of death worldwide. Efficient non-invasive blood-based biomarkers for CRC early detection and prognosis are urgently needed.
Methods: To identify novel potential plasma biomarkers, we applied a proximity extension assay (PEA), an antibody-based proteomics strategy to quantify the abundance of plasma proteins in CRC development and cancer-associated inflammation from few μL of plasma sample.
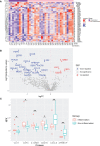
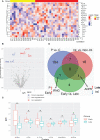
Results: Among the 690 quantified proteins, levels of 202 plasma proteins were significantly changed in CRC patients compared to age-and-sex-matched healthy subjects. We identified novel protein changes involved in Th17 activity, oncogenic pathways, and cancer-related inflammation with potential implications in the CRC diagnosis. Moreover, the interferon γ (IFNG), interleukin (IL) 32, and IL17C were identified as associated with the early stages of CRC, whereas lysophosphatidic acid phosphatase type 6 (ACP6), Fms-related tyrosine kinase 4 (FLT4), and MANSC domain-containing protein 1 (MANSC1) were correlated with the late-stages of CRC.
Discussion: Further study to characterize the newly identified plasma protein changes from larger cohorts will facilitate the identification of potential novel diagnostic, prognostic biomarkers for CRC.
Keywords: biomarker; colorectal cancer; cytokines; early detection; inflammation; plasma proteomics; prognosis; proximity extension assay.
Copyright © 2023 Urbiola-Salvador, Jabłońska, Miroszewska, Huang, Duzowska, Drężek-Chyła, Zdrenka, Śrutek, Szylberg, Jankowski, Bała, Zegarski, Nowikiewicz, Makarewicz, Adamczyk, Ambicka, Przewoźnik, Harazin-Lechowicz, Ryś, Filipowicz, Piotrowski, Dumanski, Li and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Nicholson BD, James T, East JE, Grimshaw D, Paddon M, Justice S, et al. . Experience of adopting faecal immunochemical testing to meet the NICE colorectal cancer referral criteria for low-risk symptomatic primary care patients in Oxfordshire, UK. Frontline Gastroenterol (2019) 10:347–55. doi: 10.1136/flgastro-2018-101052 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

