Preclinical investigations of the efficacy of the glutaminase inhibitor CB-839 alone and in combinations in chronic lymphocytic leukemia
- PMID: 37228498
- PMCID: PMC10203524
- DOI: 10.3389/fonc.2023.1161254
Preclinical investigations of the efficacy of the glutaminase inhibitor CB-839 alone and in combinations in chronic lymphocytic leukemia
Abstract
Introduction: Chronic lymphocytic leukemia (CLL) cells are metabolically flexible and adapt to modern anticancer treatments. Bruton tyrosine kinase (BTK) and B-cell lymphoma-2 (BCL-2) inhibitors have been widely used to treat CLL, but CLL cells become resistant to these treatments over time. CB-839 is a small-molecule glutaminase-1 (GLS-1) inhibitor that impairs glutamine use, disrupts downstream energy metabolism, and impedes the elimination of reactive oxygen species.
Methods: To investigate the in vitro effects of CB-839 on CLL cells, we tested CB-839 alone and in combination with ibrutinib, venetoclax, or AZD-5991 on the HG-3 and MEC-1 CLL cell lines and on primary CLL lymphocytes.
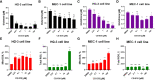
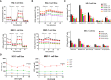
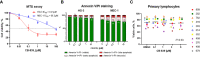
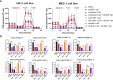
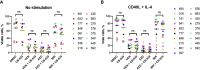
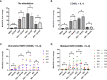
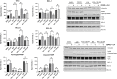
Results: We found that CB-839 caused dose-dependent decreases in GLS-1 activity and glutathione synthesis. CB-839-treated cells also showed increased mitochondrial superoxide metabolism and impaired energy metabolism, which were reflected in decreases in the oxygen consumption rate and depletion of the adenosine triphosphate pool and led to the inhibition of cell proliferation. In the cell lines, CB-839 combined with venetoclax or AZD-5991, but not with ibrutinib, demonstrated synergism with an increased apoptosis rate and cell proliferation inhibition. In the primary lymphocytes, no significant effects of CB-839 alone or in combination with venetoclax, ibrutinib, or AZD-5991 were observed.
Discussion: Our findings suggest that CB-839 has limited efficacy in CLL treatment and shows limited synergy in combination with widely used CLL drugs.
Keywords: BTK - Bruton’s tyrosine kinase; Bcl-2 inhibitor; CLL - chronic lymphoblastic leukemia; Mcl-1 inhibitor; glutamine (Gln) metabolism; ibrutinib; venetoclax.
Copyright © 2023 Timofeeva, Ayres, Baran, Santiago-O’Farrill, Bildik, Lu, Konopleva and Gandhi.
Conflict of interest statement
Previously, for other investigations, V.G. received research funding from AbbVie, Acerta, AstraZeneca, Clear Creek Bio., Gilead, Infinity, Loxo/Lilly Oncology, Pharmacyclics, and Sunesis. The remaining authors declare no competing financial interests.
Figures


Similar articles
-
Targeting Mcl-1 by AMG-176 During Ibrutinib and Venetoclax Therapy in Chronic Lymphocytic Leukemia.Front Oncol. 2022 Feb 22;12:833714. doi: 10.3389/fonc.2022.833714. eCollection 2022. Front Oncol. 2022. PMID: 35273915 Free PMC article.
-
Preclinical evaluation of combination nemtabrutinib and venetoclax in chronic lymphocytic leukemia.J Hematol Oncol. 2022 Nov 15;15(1):166. doi: 10.1186/s13045-022-01386-1. J Hematol Oncol. 2022. PMID: 36380319 Free PMC article.
-
BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia.Curr Treat Options Oncol. 2021 Jun 10;22(8):66. doi: 10.1007/s11864-021-00862-z. Curr Treat Options Oncol. 2021. PMID: 34110507 Review.
-
Bruton's tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia.Leukemia. 2017 Oct;31(10):2075-2084. doi: 10.1038/leu.2017.32. Epub 2017 Jan 23. Leukemia. 2017. PMID: 28111464 Free PMC article.
-
Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review.JAMA. 2023 Mar 21;329(11):918-932. doi: 10.1001/jama.2023.1946. JAMA. 2023. PMID: 36943212 Review.
Cited by
-
Exploiting the Achilles' heel of cancer: disrupting glutamine metabolism for effective cancer treatment.Front Pharmacol. 2024 Mar 6;15:1345522. doi: 10.3389/fphar.2024.1345522. eCollection 2024. Front Pharmacol. 2024. PMID: 38510646 Free PMC article. Review.
-
Targeting MCL-1 protein to treat cancer: opportunities and challenges.Front Oncol. 2023 Jul 31;13:1226289. doi: 10.3389/fonc.2023.1226289. eCollection 2023. Front Oncol. 2023. PMID: 37601693 Free PMC article. Review.
-
Metabolic crossroads: unravelling immune cell dynamics in gastrointestinal cancer drug resistance.Cancer Drug Resist. 2025 Feb 8;8:7. doi: 10.20517/cdr.2024.164. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40051496 Free PMC article. Review.
-
Magnetic resonance spectroscopy-based detection of response to therapy targeting glutaminolysis in lymphoma.Blood Neoplasia. 2025 Apr 15;2(3):100105. doi: 10.1016/j.bneo.2025.100105. eCollection 2025 Aug. Blood Neoplasia. 2025. PMID: 40575077 Free PMC article. No abstract available.
-
Glutamine and leukemia research: progress and clinical prospects.Discov Oncol. 2024 Aug 31;15(1):391. doi: 10.1007/s12672-024-01245-0. Discov Oncol. 2024. PMID: 39215845 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

