A comprehensive comparison of the in vitro hemocompatibility of extracorporeal centrifugal blood pumps
- PMID: 37228828
- PMCID: PMC10204736
- DOI: 10.3389/fphys.2023.1136545
A comprehensive comparison of the in vitro hemocompatibility of extracorporeal centrifugal blood pumps
Abstract
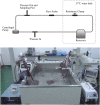
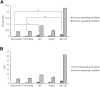
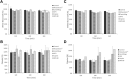
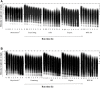
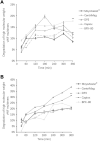
Purpose: Blood damage has been associated with patients under temporary continuous-flow mechanical circulatory support. To evaluate the side effects caused by transit blood pumping, in vitro hemocompatibility testing for blood damage in pumps is considered a necessary reference before clinical trials. Methods: The hemocompatibility of five extracorporeal centrifugal blood pumps was investigated comprehensively, including four commercial pumps (the Abbott CentriMag, the Terumo Capiox, the Medos DP3, and the Medtronic BPX-80) and a pump in development (the magAssist MoyoAssist®). In vitro, hemolysis was tested with heparinized porcine blood at nominal operating conditions (5 L/min, 160 mmHg) and extreme operating conditions (1 L/min, 290 mmHg) using a circulation flow loop. Hematology analyses concerning the blood cell counts and the degradation of high-molecular-weight von Willebrand factor (VWF) during 6-h circulation were also evaluated. Results: Comparing the in vitro hemocompatibility of blood pumps at different operations, the blood damage was significantly more severe at extreme operating conditions than that at nominal operating conditions. The performance of the five blood pumps was arranged in different orders at these two operating conditions. The results also demonstrated superior hemocompatibility of CentriMag and MoyoAssist® at two operating conditions, with overall low blood damage at hemolysis level, blood cell counts, and degradation of high-molecular-weight VWF. It suggested that magnetic bearings have an advantage in hemocompatibility compared to the mechanical bearing of blood pumps. Conclusion: Involving multiple operating conditions of blood pumps in in vitro hemocompatibility evaluation will be helpful for clinical application. In addition, the magnetically levitated centrifugal blood pump MoyoAssist® shows great potential in the future as it demonstrated good in vitro hemocompatibility.
Keywords: ECMO; blood damage; centrifugal pump; hemolysis; von Willebrand factor.
Copyright © 2023 Li, Mei, Ge, Wu, Zhong, Huan, Jiang, Hsu, Steinseifer, Dong and Zhang.
Conflict of interest statement
P-LH is the founder and CEO of magAssist, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- ASTM (2019). ASTM F1841-97 standard practice for assessment of hemolysis in continuous flow blood pumps. United States: ASTM International. 10.1520/F1841-19.clinical - DOI
-
- Bansal A., Uriel N., Colombo P. C., Narisetty K., Long J. W., Bhimaraj A., et al. (2019). Effects of a fully magnetically levitated centrifugal-flow or axial-flow left ventricular assist device on von Willebrand factor: A prospective multicenter clinical trial. J. Hear Lung Transpl. 38, 806–816. 10.1016/j.healun.2019.05.006 - DOI - PubMed
-
- Bartoli C. R., Restle D. J., Zhang D. M., Acker M. A., Atluri P. (2015). Pathologic von Willebrand factor degradation with a left ventricular assist device occurs via two distinct mechanisms: Mechanical demolition and enzymatic cleavage. J. Thorac. Cardiovasc Surg. 149, 281–289. 10.1016/j.jtcvs.2014.09.031 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

