Effectiveness of Casirivimab and Imdevimab Antibody Combination in Immunocompromised Hospitalized Patients With Coronavirus Disease 2019: A Post Hoc Analysis in a Phase 1/2/3 Double-Blind Trial
- PMID: 37229174
- PMCID: PMC10205469
- DOI: 10.1093/ofid/ofad211
Effectiveness of Casirivimab and Imdevimab Antibody Combination in Immunocompromised Hospitalized Patients With Coronavirus Disease 2019: A Post Hoc Analysis in a Phase 1/2/3 Double-Blind Trial
Abstract
Background: Individuals who are immunocompromised (IC) are at high risk for severe coronavirus disease 2019 (COVID-19).
Methods: Post hoc analyses of a double-blind trial conducted prior to Omicron (June 2020-April 2021), in hospitalized patients with COVID-19 assessed viral load, clinical outcomes, and safety of casirivimab plus imdevimab (CAS + IMD) versus placebo in IC versus overall study patients.
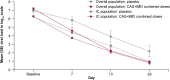
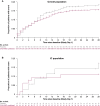
Results: Ninety-nine of 1940 (5.1%) patients were IC. IC versus overall patients were more frequently seronegative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies (68.7% vs 41.2%) and had higher median baseline viral loads (7.21 vs 6.32 log10 copies/mL). On placebo, IC versus overall patients had slower viral load declines. CAS + IMD reduced viral load in IC and overall patients; least-squares mean difference versus placebo in time-weighted average change from baseline viral load at day 7 was -0.69 (95% confidence interval [CI], -1.25 to -.14) log10 copies/mL for IC patients and -0.31 (95% CI, -.42 to -.20) log10 copies/mL for overall patients. For IC patients, the cumulative incidence of death or mechanical ventilation at day 29 was lower with CAS + IMD (11.0%) versus placebo (17.2%), consistent with overall patients (15.7% CAS + IMD vs 18.3% placebo). IC and overall patients receiving CAS + IMD exhibited similar rates of treatment-emergent adverse events (30.4% and 26.6%, respectively), grade ≥2 hypersensitivity or infusion-related reactions (1.4% and 2.5%), and deaths (8.7% and 12.2%).
Conclusions: IC patients were more likely to exhibit high viral loads and be seronegative at baseline. For susceptible SARS-CoV-2 variants, CAS + IMD reduced viral load and resulted in fewer death or mechanical ventilation events in IC and overall study patients. There were no new safety findings among IC patients. Clinical Trials Registration. NCT04426695.
Keywords: COVID-19; hospitalized; immunocompromised; monoclonal antibody.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. S. S.-K., J. M., V. M. C., A. M., M. H., S. A., R. B., and B. H. are employees/stockholders of Regeneron Pharmaceuticals, and report grants from BARDA. E. M. reports payments to his institution received from the National Institute of Allergy and Infectious Diseases/National Institutes of Health (NIH), National Institute of General Medical Sciences/NIH, SciClone Pharmaceuticals, Regeneron Pharmaceuticals, Pfizer, Chemic Labs/KODA Therapeutics, Cidara, and Leidos Biomedical Research Inc/National Cancer Institute. E. O.-O. was an employee of and holds stocks and shares in Regeneron Pharmaceuticals, and reports grants from BARDA. M. P. O. and G. A. H. are employees/stockholders of Regeneron Pharmaceuticals and report having a patent pending, which has been licensed and receiving royalties, with Regeneron Pharmaceuticals, as well as a pending patent application; and report grants from BARDA. A. T. H. is an employee/stockholder of Regeneron Pharmaceuticals, a former Pfizer employee and current stockholder, has a patent pending with Regeneron Pharmaceuticals, and reports grants from BARDA. F. M. M. reports payments to his institution received from Regeneron Pharmaceuticals, F2G, Gilead, Merck, Shire, Syneos, and the Bill & Melinda Gates Foundation; reports grants or contracts from Ansun, Chimerix, Cidara, Scynexis, Shire (a Takeda company), WHISCON, AlloVir, Amplyx, F2G, Gilead, and Merck; reports consulting fees from AlloVir, Amplyx, Avir, Emcure, F2G, Gilead, Janssen, Kyorin, Merck, Regeneron, ReViral, Symbio, United Medical, ADMA Biologics, Astellas, Behring, Cidara, Octapharma, Partner Therapeutics, Shionogi, Shire (a Takeda company), and Siemens Healthineers; reports payment for honoraria from Basilea, Merck, and Merck Sharp & Dohme Europe; and reports grants from BARDA. E. F.-N. was an employee of and holds stocks and shares in Regeneron Pharmaceuticals and reports having a patent pending, which has been licensed and receiving royalties from Regeneron Pharmaceuticals, as well as a pending patent application; and reports grants from BARDA. J. D. H. is an employee/stockholder of Regeneron Pharmaceuticals and has a patent pending, which has been licensed and receiving royalties, with Regeneron Pharmaceuticals. D. M. W. was an employee of and holds stocks and shares in Regeneron Pharmaceuticals and reports having a pending patent application; and reports grants from BARDA.
Figures

References
-
- Harpaz R, Dahl RM, Dooling KL. Prevalence of immunosuppression among US adults, 2013. JAMA 2016; 316:2547–8. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

