Efficacy and safety of vedolizumab in the treatment of patients with inflammatory bowel disease: A systematic review and meta‑analysis of randomized controlled trials
- PMID: 37229320
- PMCID: PMC10203751
- DOI: 10.3892/etm.2023.11997
Efficacy and safety of vedolizumab in the treatment of patients with inflammatory bowel disease: A systematic review and meta‑analysis of randomized controlled trials
Abstract
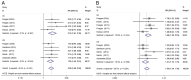
Few studies have thoroughly assessed the efficacy and safety of vedolizumab (VDZ) in the treatment of inflammatory bowel disease (IBD). Therefore, this systematic review and meta-analysis was performed to further evaluate this association. PubMed, Embase, and the Cochrane databases were searched until April 2022. Randomized controlled trials (RCTs) evaluating the efficacy and safety of VDZ in the treatment of IBD were included. The risk ratio (RR) and 95% confidence intervals (CI) were estimated for each outcome using a random effects model. A total of 12 RCTs, including 4,865 patients, met the inclusion criteria. In the induction phase, VDZ was more effective than placebo for patients with ulcerative colitis and Crohn's disease (CD) in clinical remission (RR=2.09; 95% CI=1.66-2.62) and clinical response (RR=1.54; 95% CI=1.34-1.78). In the maintenance therapy group, VDZ reached higher clinical remission (RR=1.98; 95% CI=1.58-2.49) and clinical response (RR=1.78; 95% CI=1.40-2.26) rates compared with the placebo group. VDZ particularly improved clinical remission (RR=2.07; 95% CI=1.48-2.89) and clinical response (RR=1.84; 95% CI=1.54-2.21) in patients with TNF antagonist failure. In terms of corticosteroid-free remission, VDZ was also more effective than placebo in patients with IBD (RR=1.98; 95% CI=1.51-2.59). In Crohn's patients, VDZ was more effective than placebo in terms of mucosal healing (RR=1.78; 95% CI=1.27-2.51). With respect to adverse events, VDZ significantly reduced the risk of IBD exacerbation compared with the placebo (RR=0.60; 95% CI=0.39-0.93; P=0.023). However, when compared with the placebo, VDZ increased the risk of nasopharyngitis in patients with CD (RR=1.77; 95% CI=1.01-3.10; P=0.045). No significant differences in other adverse events were observed. Although there might be underlying risk, such as selection bias, in the present study it can be safely concluded that VDZ is a safe and effective biological agent for IBD, particularly for patients with TNF antagonist failure.
Keywords: inflammatory bowel disease; meta-analysis; randomized controlled trials; vedolizumab.
Copyright: © Tang et al.
Conflict of interest statement
The authors declare that they have no competing interests.
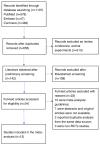
Figures


Similar articles
-
Vedolizumab subcutaneous formulation maintenance therapy for patients with IBD: a systematic review and meta-analysis.Therap Adv Gastroenterol. 2023 Apr 27;16:17562848231166227. doi: 10.1177/17562848231166227. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 37124368 Free PMC article. Review.
-
Effectiveness and safety outcomes after long-term (54 weeks) vedolizumab therapy for Crohn's disease: a prospective, real-world observational study including patient-reported outcomes (POLONEZ II).Therap Adv Gastroenterol. 2024 Nov 20;17:17562848241293938. doi: 10.1177/17562848241293938. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39575158 Free PMC article.
-
Comparing the Efficacy and Safety of Adalimumab and Vedolizumab in Treating Moderate to Severe Crohn's Disease and Ulcerative Colitis.Gastroenterology Res. 2023 Dec;16(6):289-306. doi: 10.14740/gr1664. Epub 2023 Nov 3. Gastroenterology Res. 2023. PMID: 38186583 Free PMC article.
-
Anti-IL-12/23p40 antibodies for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2019 Dec 12;12(12):CD012804. doi: 10.1002/14651858.CD012804.pub2. Cochrane Database Syst Rev. 2019. PMID: 31828765 Free PMC article.
-
Adalimumab for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2020 May 16;5(5):CD012877. doi: 10.1002/14651858.CD012877.pub2. Cochrane Database Syst Rev. 2020. PMID: 32413933 Free PMC article.
Cited by
-
HLA Class I Expression Is Associated with DNA Damage and Immune Cell Infiltration into Dysplastic and Neoplastic Lesions in Ulcerative Colitis.Int J Mol Sci. 2023 Sep 4;24(17):13648. doi: 10.3390/ijms241713648. Int J Mol Sci. 2023. PMID: 37686454 Free PMC article.
References
-
- Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68 (Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
