2023 Consensus of Taiwan Society of Cardiology on the Pharmacological Treatment of Chronic Heart Failure
- PMID: 37229331
- PMCID: PMC10203721
- DOI: 10.6515/ACS.202305_39(3).20230301A
2023 Consensus of Taiwan Society of Cardiology on the Pharmacological Treatment of Chronic Heart Failure
Abstract
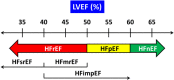
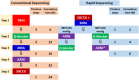
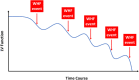
The prevalence of heart failure is increasing, causing a tremendous burden on health care systems around the world. Although mortality rate of heart failure has been significantly reduced by several effective agents in the past 3 decades, yet it remains high in observational studies. More recently, several new classes of drugs emerged with significant efficacy in reducing mortality and hospitalization in chronic heart failure with reduced ejection fraction (HFrEF) and preserved ejection fraction (HFpEF). To integrate these effective therapies and prioritize them in the management of Asian patients, Taiwan Society of Cardiology has recently appointed a working group to formulate a consensus of pharmacological treatment in patients with chronic heart failure. Based on most updated information, this consensus provides rationales for prioritization, rapid sequencing, and in-hospital initiation of both foundational and additional therapies for patients with chronic heart failure.
Keywords: Asia; Chronic heart failure; Consensus; Foundational therapy; Left ventricular ejection fraction.
Conflict of interest statement
Dr. C.-E. Chiang has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Menarini, MSD, Novartis, Pfizer, and Sanofi. Dr. C.-L. Hung has received honorarium from Astrazeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Pfizer, and Sanofi. Dr. Y.-W. Wu has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Pfizer, Sanofi, and Takeda. Dr. T.-H. Lin has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Pfizer, Sanofi, Bayer, Viatris, Menariri, Tanabe, and Takeda. Dr. K.-C. Ueng has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Merck Sharp and Dohme, Novartis, Pfizer, and Sanofi. Dr. S.-H. Sung has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, Sanofi, Abbott, and Edward. Dr. C.-K. Wu has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, Sanofi, and Abbott. Dr. T.-H. Chao has received honorarium from Bayer, AstraZeneca, Eli Lilly, Boehringer Ingelheim, Daiichi-Sankyo, Tanabe Taiwan, and Novartis. Dr. Y.-H. Lin has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Novartis, Pfizer, and Sanofi. Dr. M. YC Chen has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, and Pfizer. Dr. P.-L. Lin has received honorarium from AstraZeneca, Abbott, Boehringer Ingelheim, Bayer, Daiichi-Sankyo, Eli Lilly, Novartis, Novo Nordisk, Pfizer, and Sanofi. Dr. T.-F. Chao has received speaker honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, and Sanofi. Dr. H.-M. Cheng has received honorarium from AstraZeneca, Pfizer, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, SERVIER, Sanofi, Takeda, and Eli Lilly. Dr. M.-E. Liu has received honorarium from Abbott, AstraZeneca, Bayer, Biotronik, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Medtronic, MSD, Novartis, Pfizer, and Sanofi. Dr. H.-I. Yeh has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, MSD, Novartis, Novo Nordisk, Pfizer, and Sanofi. Dr. Y.-H. Li has received honorarium from AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Eli Lilly, Novartis, Pfizer, and Sanofi. Dr. I.-C. Hsieh has received honorarium from AstraZeneca, Boehringer Ingelheim, Novartis, Bayer, MSD, Sanofi, Daiichi Sankyo, Pfizer, and Eli Lilly. Dr. C.-C. Wang has received honorarium from AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, Novartis, and Pfizer. Dr. C.-H. Chen reports honoraria from Novartis and Daiichi Sankyo. Dr. P.-H. Chu has received honorarium from AstraZeneca. Dr. S.-J. Yeh has received honorarium from Tanabe. Dr. W.-J. Chen has received honorarium from Astrazeneca, Boehringer Ingelheim, Daiichi-Sankyo, MSD, Novartis, Pfizer, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380:539–548. - PubMed
-
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2020;384:117–128. - PubMed
-
- Mebazaa A, Davison B, Chioncel O, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400:1938–1952. - PubMed
-
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
