The effect of the subthreshold oscillation induced by the neurons' resonance upon the electrical stimulation-dependent instability
- PMID: 37229430
- PMCID: PMC10203711
- DOI: 10.3389/fnins.2023.1178606
The effect of the subthreshold oscillation induced by the neurons' resonance upon the electrical stimulation-dependent instability
Abstract
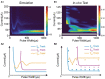
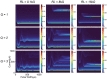
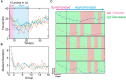
Repetitive electrical nerve stimulation can induce a long-lasting perturbation of the axon's membrane potential, resulting in unstable stimulus-response relationships. Despite being observed in electrophysiology, the precise mechanism underlying electrical stimulation-dependent (ES-dependent) instability is still an open question. This study proposes a model to reveal a facet of this problem: how threshold fluctuation affects electrical nerve stimulations. This study proposes a new method based on a Circuit-Probability theory (C-P theory) to reveal the interlinkages between the subthreshold oscillation induced by neurons' resonance and ES-dependent instability of neural response. Supported by in-vivo studies, this new model predicts several key characteristics of ES-dependent instability and proposes a stimulation method to minimize the instability. This model provides a powerful tool to improve our understanding of the interaction between the external electric field and the complexity of the biophysical characteristics of axons.
Keywords: Circuit-Probability theory; neural modeling; neural oscillation; subthreshold oscillation; threshold fluctuation.
Copyright © 2023 Yu, Yue, Guo, Liu, Zhang, Khademi, Zhou, Xu, Song, Wu, Liu, Tai, Yu and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor XS declared a shared affiliation with the author YZ at the time of review.
Figures









References
LinkOut - more resources
Full Text Sources
Miscellaneous

