Senescence: a double-edged sword in beta-cell health and failure?
- PMID: 37229454
- PMCID: PMC10203573
- DOI: 10.3389/fendo.2023.1196460
Senescence: a double-edged sword in beta-cell health and failure?
Abstract
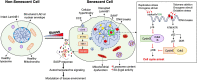
Cellular senescence is a complex process marked by permanent cell-cycle arrest in response to a variety of stressors, and acts as a safeguard against the proliferation of damaged cells. Senescence is not only a key process underlying aging and development of many diseases, but has also been shown to play a vital role in embryogenesis as well as tissue regeneration and repair. In context of the pancreatic beta-cells, that are essential for maintaining glucose homeostasis, replicative senescence is responsible for the age-related decline in regenerative capacity. Stress induced premature senescence is also a key early event underlying beta-cell failure in both type 1 and type 2 diabetes. Targeting senescence has therefore emerged as a promising therapeutic avenue for diabetes. However, the molecular mechanisms that mediate the induction of beta-cell senescence in response to various stressors remain unclear. Nor do we know if senescence plays any role during beta-cell growth and development. In this perspective, we discuss the significance of senescence in beta-cell homeostasis and pathology and highlight emerging directions in this area that warrant our attention.
Keywords: aging; beta cells; diabetes; differentiation; epigenetics; maturation; proliferation; senescence.
Copyright © 2023 Varghese and Dhawan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

