Transit Amplifying Cells (TACs): a still not fully understood cell population
- PMID: 37229487
- PMCID: PMC10203484
- DOI: 10.3389/fbioe.2023.1189225
Transit Amplifying Cells (TACs): a still not fully understood cell population
Abstract
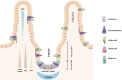
Maintenance of tissue homeostasis and tissue regeneration after an insult are essential functions of adult stem cells (SCs). In adult tissues, SCs proliferate at a very slow rate within "stem cell niches", but, during tissue development and regeneration, before giving rise to differentiated cells, they give rise to multipotent and highly proliferative cells, known as transit-amplifying cells (TACs). Although differences exist in diverse tissues, TACs are not only a transitory phase from SCs to post-mitotic cells, but they also actively control proliferation and number of their ancestor SCs and proliferation and differentiation of their progeny toward tissue specific functional cells. Autocrine signals and negative and positive feedback and feedforward paracrine signals play a major role in these controls. In the present review we will consider the generation and the role played by TACs during development and regeneration of lining epithelia characterized by a high turnover including epidermis and hair follicles, ocular epithelial surfaces, and intestinal mucosa. A comparison between these different tissues will be made. There are some genes and molecular pathways whose expression and activation are common to most TACs regardless their tissue of origin. These include, among others, Wnt, Notch, Hedgehog and BMP pathways. However, the response to these molecular signals can vary in TACs of different tissues. Secondly, we will consider cultured cells derived from tissues of mesodermal origin and widely adopted for cell therapy treatments. These include mesenchymal stem cells and dedifferentiated chondrocytes. The possible correlation between cell dedifferentiation and reversion to a transit amplifying cell stage will be discussed.
Keywords: cell dedifferentiation; cell reprogramming; cultured cells; hair follicle; intestinal crypt; stem cells; tissue differentiation; tissue regeneration.
Copyright © 2023 Cancedda and Mastrogiacomo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

