Senescence induces fundamental changes in the secretome of mesenchymal stromal cells (MSCs): implications for the therapeutic use of MSCs and their derivates
- PMID: 37229499
- PMCID: PMC10203235
- DOI: 10.3389/fbioe.2023.1148761
Senescence induces fundamental changes in the secretome of mesenchymal stromal cells (MSCs): implications for the therapeutic use of MSCs and their derivates
Abstract
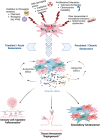
Mesenchymal stromal cells (MSCs) are a heterogeneous population containing multipotent adult stem cells with a multi-lineage differentiation capacity, which differentiated into mesodermal derivatives. MSCs are employed for therapeutic purposes and several investigations have demonstrated that the positive effects of MSC transplants are due to the capacity of MSCs to modulate tissue homeostasis and repair via the activity of their secretome. Indeed, the MSC-derived secretomes are now an alternative strategy to cell transplantation due to their anti-inflammatory, anti-apoptotic, and regenerative effects. The cellular senescence is a dynamic process that leads to permanent cell cycle arrest, loss of healthy cells' physiological functions and acquiring new activities, which are mainly accrued through the release of many factors, indicated as senescence-associated secretory phenotype (SASP). The senescence occurring in stem cells, such as those present in MSCs, may have detrimental effects on health since it can undermine tissue homeostasis and repair. The analysis of MSC secretome is important either for the MSC transplants and for the therapeutic use of secretome. Indeed, the secretome of MSCs, which is the main mechanism of their therapeutic activity, loses its beneficial functions and acquire negative pro-inflammatory and pro-aging activities when MSCs become senescent. When MSCs or their derivatives are planned to be used for therapeutic purposes, great attention must be paid to these changes. In this review, we analyzed changes occurring in MSC secretome following the switch from healthy to senescence status.
Keywords: SASP; aging; mesenchymal stromal cells (MSC); secretome; senescence.
Copyright © 2023 Siraj, Galderisi and Alessio.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity.Oncotarget. 2015 Nov 24;6(37):39482-92. doi: 10.18632/oncotarget.5430. Oncotarget. 2015. PMID: 26498687 Free PMC article.
-
Role of mesenchymal stromal cell secretome on recovery from cellular senescence: an overview.Cytotherapy. 2025 Apr;27(4):422-437. doi: 10.1016/j.jcyt.2024.11.014. Epub 2024 Nov 22. Cytotherapy. 2025. PMID: 39674933 Review.
-
Hydrogel Culture Surface Stiffness Modulates Mesenchymal Stromal Cell Secretome and Alters Senescence.Tissue Eng Part A. 2020 Dec;26(23-24):1259-1271. doi: 10.1089/ten.tea.2020.0030. Epub 2020 Jul 6. Tissue Eng Part A. 2020. PMID: 32628570
-
Quantifying Senescence-Associated Phenotypes in Primary Multipotent Mesenchymal Stromal Cell Cultures.Methods Mol Biol. 2019;2045:93-105. doi: 10.1007/7651_2019_217. Methods Mol Biol. 2019. PMID: 31020633
-
Cellular expansion of MSCs: Shifting the regenerative potential.Aging Cell. 2023 Jan;22(1):e13759. doi: 10.1111/acel.13759. Epub 2022 Dec 19. Aging Cell. 2023. PMID: 36536521 Free PMC article. Review.
Cited by
-
Antimicrobial activity of adipose-derived mesenchymal stromal cell secretome against methicillin-resistant Staphylococcus aureus.Stem Cell Res Ther. 2025 Jan 23;16(1):21. doi: 10.1186/s13287-025-04138-3. Stem Cell Res Ther. 2025. PMID: 39849590 Free PMC article.
-
Human umbilical cord-derived mesenchymal stromal cell exosomes ameliorate aging-associated skeletal muscle atrophy and dysfunction in SAMP10 mice.Stem Cell Res Ther. 2025 Jul 28;16(1):410. doi: 10.1186/s13287-025-04477-1. Stem Cell Res Ther. 2025. PMID: 40722197 Free PMC article.
-
IGFBP5 is released by senescent cells and is internalized by healthy cells, promoting their senescence through interaction with retinoic receptors.Cell Commun Signal. 2024 Feb 13;22(1):122. doi: 10.1186/s12964-024-01469-1. Cell Commun Signal. 2024. PMID: 38351010 Free PMC article.
-
Mesenchymal stromal cell exosomes for drug delivery of prostate cancer treatments: a review.Stem Cell Res Ther. 2025 Jan 23;16(1):18. doi: 10.1186/s13287-025-04133-8. Stem Cell Res Ther. 2025. PMID: 39849570 Free PMC article. Review.
-
Fucoxanthin diminishes oxidative stress damage in human placenta-derived mesenchymal stem cells through the PI3K/Akt/Nrf-2 pathway.Sci Rep. 2023 Dec 27;13(1):22974. doi: 10.1038/s41598-023-49751-5. Sci Rep. 2023. PMID: 38151503 Free PMC article.
References
-
- Alessio N., Aprile D., Squillaro T., Di Bernardo G., Finicelli M., Melone M. A., et al. (2019a). The senescence-associated secretory phenotype (SASP) from mesenchymal stromal cells impairs growth of immortalized prostate cells but has no effect on metastatic prostatic cancer cells. Aging (Albany NY) 11, 5817–5828. 10.18632/aging.102172 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

