Supramolecular Polymer-Nanomedicine Hydrogel Loaded with Tumor Associated Macrophage-Reprogramming polyTLR7/8a Nanoregulator for Enhanced Anti-Angiogenesis Therapy of Orthotopic Hepatocellular Carcinoma
- PMID: 37229748
- PMCID: PMC10401096
- DOI: 10.1002/advs.202300637
Supramolecular Polymer-Nanomedicine Hydrogel Loaded with Tumor Associated Macrophage-Reprogramming polyTLR7/8a Nanoregulator for Enhanced Anti-Angiogenesis Therapy of Orthotopic Hepatocellular Carcinoma
Abstract
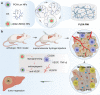
Anti-angiogenic therapies targeting inhibition of vascular endothelial growth factor (VEGF) pathway show clinical benefit in hypervascular hepatocellular carcinoma (HCC) tumors. However, HCC expresses massive pro-angiogenic factors in the tumor microenvironment (TME) in response to anti-angiogenic therapy, recruiting tumor-associated macrophages (TAMs), leading to revascularization and tumor progression. To regulate cell types in TME and promote the therapeutic efficiency of anti-angiogenic therapy, a supramolecular hydrogel drug delivery system (PLDX-PMI) co-assembled by anti-angiogenic nanomedicines (PCN-Len nanoparticles (NPs)) and oxidized dextran (DX), and loaded with TAMs-reprogramming polyTLR7/8a nanoregulators (p(Man-IMDQ) NRs) is developed for orthotopic liver cancer therapy. PCN-Len NPs target tyrosine kinases of vascular endothelial cells and blocked VEGFR signaling pathway. p(Man-IMDQ) NRs repolarize pro-angiogenic M2-type TAMs into anti-angiogenic M1-type TAMs via mannose-binding receptors, reducing the secretion of VEGF, which further compromised the migration and proliferation of vascular endothelial cells. On highly malignant orthotopic liver cancer Hepa1-6 model, it is found that a single administration of the hydrogel formulation significantly decreases tumor microvessel density, promotes tumor vascular network maturation, and reduces M2-subtype TAMs, thereby effectively inhibiting tumor progression. Collectively, findings in this work highlight the great significance of TAMs reprogramming in enhancing anti-angiogenesis treatment for orthotopic HCC, and provides an advanced hydrogel delivery system-based synergistic approach for tumor therapy.
Keywords: anti-angiogenic therapy; nanoregulator; orthotopic hepatocellular carcinoma; supramolecular hydrogel; tumor microenvironment.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Angiogenesis in hepatocellular carcinoma: mechanisms and anti-angiogenic therapies.Cancer Biol Med. 2023 Jan 12;20(1):25-43. doi: 10.20892/j.issn.2095-3941.2022.0449. Cancer Biol Med. 2023. PMID: 36647777 Free PMC article. Review.
-
Repolarization of macrophages to improve sorafenib sensitivity for combination cancer therapy.Acta Biomater. 2023 May;162:98-109. doi: 10.1016/j.actbio.2023.03.014. Epub 2023 Mar 16. Acta Biomater. 2023. PMID: 36931417
-
β,β-Dimethylacrylalkannin, a Natural Naphthoquinone, Inhibits the Growth of Hepatocellular Carcinoma Cells by Modulating Tumor-Associated Macrophages.Molecules. 2024 Aug 20;29(16):3919. doi: 10.3390/molecules29163919. Molecules. 2024. PMID: 39202998 Free PMC article.
-
Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma.Cancer Med. 2018 Sep;7(9):4570-4583. doi: 10.1002/cam4.1664. Epub 2018 Aug 14. Cancer Med. 2018. PMID: 30109780 Free PMC article.
-
Targeting tumor associated macrophages in hepatocellular carcinoma.Biochem Pharmacol. 2022 May;199:114990. doi: 10.1016/j.bcp.2022.114990. Epub 2022 Mar 11. Biochem Pharmacol. 2022. PMID: 35288152 Review.
Cited by
-
Nanomaterials modulate tumor-associated macrophages for the treatment of digestive system tumors.Bioact Mater. 2024 Mar 20;36:376-412. doi: 10.1016/j.bioactmat.2024.03.003. eCollection 2024 Jun. Bioact Mater. 2024. PMID: 38544737 Free PMC article. Review.
-
Hydrogel Crosslinked with Nanoparticles for Prevention of Surgical Hemorrhage and Recurrence of Hepatocellular Carcinoma.Adv Sci (Weinh). 2024 Mar;11(9):e2305508. doi: 10.1002/advs.202305508. Epub 2023 Dec 25. Adv Sci (Weinh). 2024. PMID: 38145957 Free PMC article.
-
Targeted Drug Delivery Strategies for the Treatment of Hepatocellular Carcinoma.Molecules. 2024 Sep 16;29(18):4405. doi: 10.3390/molecules29184405. Molecules. 2024. PMID: 39339402 Free PMC article. Review.
-
Nanotherapeutics for Macrophage Network Modulation in Tumor Microenvironments: Targets and Tools.Int J Nanomedicine. 2024 Dec 19;19:13615-13651. doi: 10.2147/IJN.S491573. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39717515 Free PMC article. Review.
-
Multifunctionally disordered TiO2 nanoneedles prevent periprosthetic infection and enhance osteointegration by killing bacteria and modulating the osteoimmune microenvironment.Theranostics. 2024 Sep 16;14(15):6016-6035. doi: 10.7150/thno.98219. eCollection 2024. Theranostics. 2024. PMID: 39346538 Free PMC article.
References
-
- Morse M. A., Sun W., Kim R., He A. R., Abada P. B., Mynderse M., Finn R. S., Clin. Cancer Res. 2019, 25, 912. - PubMed
-
- a) Llovet J. M., Castet F., Heikenwalder M., Maini M. K., Mazzaferro V., Pinato D. J., Pikarsky E., Zhu A. X., Finn R. S., Nat. Rev. Clin. Oncol. 2022, 19, 151; - PubMed
- b) Finn R. S., Qin S., Ikeda M., Galle P. R., Ducreux M., Kim T. Y., Kudo M., Breder V., Merle P., Kaseb A. O., Li D., Verret W., Xu D. Z., Hernandez S., Liu J., Huang C., Mulla S., Wang Y., Lim H. Y., Zhu A. X., Cheng A. L., I. M. Investigators , N. Engl. J. Med. 2020, 382, 1894. - PubMed
-
- a) Adachi Y., Matsuki M., Watanabe H., Takase K., Kodama K., Matsui J., Funahashi Y., Nomoto K., Cancer Invest. 2019, 37, 185; - PubMed
- b) Llovet J. M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J. F., de Oliveira A. C., Santoro A., Raoul J. L., Forner A., Schwartz M., Porta C., Zeuzem S., Bolondi L., Greten T. F., Galle P. R., Seitz J. F., Borbath I., Haussinger D., Giannaris T., Shan M., Moscovici M., Voliotis D., Bruix J., S. I. S. Group , N. Engl. J. Med. 2008, 359, 378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
