Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China
- PMID: 37229831
- PMCID: PMC10193775
- DOI: 10.1016/j.ejmech.2023.115503
Bench-to-bedside: Innovation of small molecule anti-SARS-CoV-2 drugs in China
Abstract
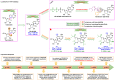
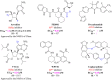
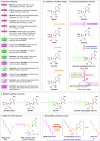
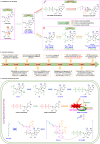
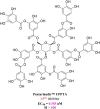
The ongoing COVID-19 pandemic has resulted in millions of deaths globally, highlighting the need to develop potent prophylactic and therapeutic strategies against SARS-CoV-2. Small molecule inhibitors (remdesivir, Paxlovid, and molnupiravir) are essential complements to vaccines and play important roles in clinical treatment of SARS-CoV-2. Many advances have been made in development of anti-SARS-CoV-2 inhibitors in China, but progress in discovery and characterization of pharmacological activity, antiviral mechanisms, and clinical efficacy are limited. We review development of small molecule anti-SARS-CoV-2 drugs (azvudine [approved by the NMPA of China on July 25, 2022], VV116 [approved by the NMPA of China on January 29, 2023], FB2001, WPV01, pentarlandir, and cepharanthine) in China and summarize their pharmacological activity, potential mechanisms of action, clinical trials and use, and important milestones in their discovery. The role of structural biology in drug development is also reviewed. Future studies should focus on development of diverse second-generation inhibitors with excellent oral bioavailability, superior plasma half-life, increased antiviral activity against SARS-CoV-2 and its variants, high target specificity, minimal side effects, reduced drug-drug interactions, and improved lung histopathology.
Keywords: Azvudine; COVID‐19; Cepharanthine; FB2001; SARS‐CoV‐2; VV116.
Copyright © 2023 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- World Health Organization WHO coronavirus (COVID-19) Dashboard. https://covid19.who.int/
-
- Wong C.K., Au I.C., Lau K.T., Lau E.H., Cowling B.J., Leung G.M. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–1222. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

