Effect of Aging and a Dual Orexin Receptor Antagonist on Sleep Architecture and Non-REM Oscillations Including an REM Behavior Disorder Phenotype in the PS19 Mouse Model of Tauopathy
- PMID: 37230765
- PMCID: PMC10286944
- DOI: 10.1523/JNEUROSCI.1828-22.2023
Effect of Aging and a Dual Orexin Receptor Antagonist on Sleep Architecture and Non-REM Oscillations Including an REM Behavior Disorder Phenotype in the PS19 Mouse Model of Tauopathy
Abstract
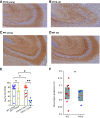
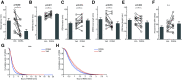
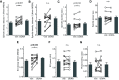
The impact of tau pathology on sleep microarchitecture features, including slow oscillations, spindles, and their coupling, has been understudied, despite the proposed importance of these electrophysiological features toward learning and memory. Dual orexin receptor antagonists (DORAs) are known to promote sleep, but whether and how they affect sleep microarchitecture in the setting of tauopathy is unknown. In the PS19 mouse model of tauopathy MAPT (microtubule-associated protein tau) P301S (both male and female), young PS19 mice 2-3 months old show a sleep electrophysiology signature with markedly reduced spindle duration and power and elevated slow oscillation (SO) density compared with littermate controls, although there is no significant tau hyperphosphorylation, tangle formation, or neurodegeneration at this age. With aging, there is evidence for sleep disruption in PS19 mice, characterized by reduced REM duration, increased non-REM and REM fragmentation, and more frequent brief arousals at the macrolevel and reduced spindle density, SO density, and spindle-SO coupling at the microlevel. In ∼33% of aged PS19 mice, we unexpectedly observed abnormal goal-directed behaviors in REM, including mastication, paw grasp, and forelimb/hindlimb extension, seemingly consistent with REM behavior disorder (RBD). Oral administration of DORA-12 in aged PS19 mice increased non-REM and REM duration, albeit with shorter bout lengths, and increased spindle density, spindle duration, and SO density without change to spindle-SO coupling, power in either the SO or spindle bands, or the arousal index. We observed a significant effect of DORA-12 on objective measures of RBD, thereby encouraging future exploration of DORA effects on sleep-mediated cognition and RBD treatment.SIGNIFICANCE STATEMENT The specific effect of tauopathy on sleep macroarchitecture and microarchitecture throughout aging remains unknown. Our key findings include the following: (1) the identification of a sleep EEG signature constituting an early biomarker of impending tauopathy; (2) sleep physiology deteriorates with aging that are also markers of off-line cognitive processing; (3) the novel observation that dream enactment behaviors reminiscent of RBD occur, likely the first such observation in a tauopathy model; and (4) a dual orexin receptor antagonist is capable of restoring several of the sleep macroarchitecture and microarchitecture abnormalities.
Keywords: DORA; REM behavior disorder; sleep; slow oscillations; spindles; tauopathy.
Copyright © 2023 the authors.
Figures




Comment in
-
Unraveling the Mechanisms Underlying Disordered Sleep in Alzheimer's Disease.J Neurosci. 2023 Nov 22;43(47):7899-7901. doi: 10.1523/JNEUROSCI.1440-23.2023. J Neurosci. 2023. PMID: 37993277 Free PMC article. No abstract available.
Similar articles
-
Orexin 2 receptor antagonism sex-dependently improves sleep/wakefulness and cognitive performance in tau transgenic mice.Br J Pharmacol. 2024 Jan;181(1):87-106. doi: 10.1111/bph.16212. Epub 2023 Sep 8. Br J Pharmacol. 2024. PMID: 37553894
-
Orexin OX2 Receptor Antagonists as Sleep Aids.Curr Top Behav Neurosci. 2017;33:105-136. doi: 10.1007/7854_2016_47. Curr Top Behav Neurosci. 2017. PMID: 27909987 Review.
-
BCI-838, an orally active mGluR2/3 receptor antagonist pro-drug, rescues learning behavior deficits in the PS19 MAPTP301S mouse model of tauopathy.Neurosci Lett. 2023 Feb 16;797:137080. doi: 10.1016/j.neulet.2023.137080. Epub 2023 Jan 16. Neurosci Lett. 2023. PMID: 36657633 Free PMC article.
-
Differential sleep-promoting effects of dual orexin receptor antagonists and GABAA receptor modulators.BMC Neurosci. 2014 Sep 22;15:109. doi: 10.1186/1471-2202-15-109. BMC Neurosci. 2014. PMID: 25242351 Free PMC article.
-
Non-REM sleep electrophysiology in REM sleep behaviour disorder: A narrative mini-review.Neurosci Biobehav Rev. 2022 Nov;142:104909. doi: 10.1016/j.neubiorev.2022.104909. Epub 2022 Oct 10. Neurosci Biobehav Rev. 2022. PMID: 36228927 Review.
Cited by
-
Dual orexin receptor antagonists as promising therapeutics for Alzheimer's disease.NPJ Biol Timing Sleep. 2025;2(1):11. doi: 10.1038/s44323-025-00025-5. Epub 2025 Mar 8. NPJ Biol Timing Sleep. 2025. PMID: 40066297 Free PMC article. Review.
-
Influence of sleep on physiological systems in atherosclerosis.Nat Cardiovasc Res. 2024 Nov;3(11):1284-1300. doi: 10.1038/s44161-024-00560-7. Epub 2024 Nov 8. Nat Cardiovasc Res. 2024. PMID: 39528718 Free PMC article. Review.
-
Sleep Disruption Precedes Forebrain Synaptic Tau Burden and Contributes to Cognitive Decline in a Sex-Dependent Manner in the P301S Tau Transgenic Mouse Model.eNeuro. 2024 Jun 26;11(6):ENEURO.0004-24.2024. doi: 10.1523/ENEURO.0004-24.2024. Print 2024 Jun. eNeuro. 2024. PMID: 38858068 Free PMC article.
-
Unraveling the Mechanisms Underlying Disordered Sleep in Alzheimer's Disease.J Neurosci. 2023 Nov 22;43(47):7899-7901. doi: 10.1523/JNEUROSCI.1440-23.2023. J Neurosci. 2023. PMID: 37993277 Free PMC article. No abstract available.
-
Reduction of orexin-expressing neurons and a unique sleep phenotype in the Tg-SwDI mouse model of Alzheimer's disease.Front Aging Neurosci. 2025 Feb 4;17:1529769. doi: 10.3389/fnagi.2025.1529769. eCollection 2025. Front Aging Neurosci. 2025. PMID: 39968126 Free PMC article.
References
-
- Arnulf I, Merino-Andreu M, Bloch F, Konofal E, Vidailhet M, Cochen V, Derenne J-P, Agid Y (2005) REM sleep behavior disorder and REM sleep without atonia in patients with progressive supranuclear palsy. Sleep 28:349–354. - PubMed
-
- Audrain M, Haure-Mirande J-V, Wang M, Kim SH, Fanutza T, Chakrabarty P, Fraser P, St George-Hyslop PH, Golde TE, Blitzer RD, Schadt EE, Zhang B, Ehrlich ME, Gandy S (2019) Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau. Mol Psychiatry 24:1383–1397. 10.1038/s41380-018-0258-3 - DOI - PMC - PubMed
-
- Bugiani O, Murrell JR, Giaccone G, Hasegawa M, Ghigo G, Tabaton M, Morbin M, Primavera A, Carella F, Solaro C, Grisoli M, Savoiardo M, Spillantini MG, Tagliavini F, Goedert M, Ghetti B (1999) Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in tau. J Neuropathol Exp Neurol 58:667–677. 10.1097/00005072-199906000-00011 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
