Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome
- PMID: 37230968
- PMCID: PMC10211313
- DOI: 10.1038/s41392-023-01496-3
Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome
Abstract
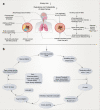
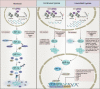
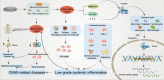
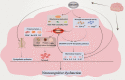
Obstructive sleep apnea syndrome (OSAS) is a common breathing disorder in sleep in which the airways narrow or collapse during sleep, causing obstructive sleep apnea. The prevalence of OSAS continues to rise worldwide, particularly in middle-aged and elderly individuals. The mechanism of upper airway collapse is incompletely understood but is associated with several factors, including obesity, craniofacial changes, altered muscle function in the upper airway, pharyngeal neuropathy, and fluid shifts to the neck. The main characteristics of OSAS are recurrent pauses in respiration, which lead to intermittent hypoxia (IH) and hypercapnia, accompanied by blood oxygen desaturation and arousal during sleep, which sharply increases the risk of several diseases. This paper first briefly describes the epidemiology, incidence, and pathophysiological mechanisms of OSAS. Next, the alterations in relevant signaling pathways induced by IH are systematically reviewed and discussed. For example, IH can induce gut microbiota (GM) dysbiosis, impair the intestinal barrier, and alter intestinal metabolites. These mechanisms ultimately lead to secondary oxidative stress, systemic inflammation, and sympathetic activation. We then summarize the effects of IH on disease pathogenesis, including cardiocerebrovascular disorders, neurological disorders, metabolic diseases, cancer, reproductive disorders, and COVID-19. Finally, different therapeutic strategies for OSAS caused by different causes are proposed. Multidisciplinary approaches and shared decision-making are necessary for the successful treatment of OSAS in the future, but more randomized controlled trials are needed for further evaluation to define what treatments are best for specific OSAS patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

