Prediction and experimental evidence of different growth phases of the Podospora anserina hyphal network
- PMID: 37231023
- PMCID: PMC10213032
- DOI: 10.1038/s41598-023-35327-w
Prediction and experimental evidence of different growth phases of the Podospora anserina hyphal network
Abstract
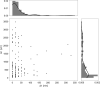
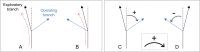
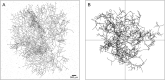
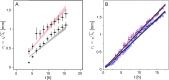
Under ideal conditions, the growth of the mycelial network of a filamentous fungus is monotonous, showing an ever increasing complexity with time. The components of the network growth are very simple and based on two mechanisms: the elongation of each hypha, and their multiplication by successive branching. These two mechanisms are sufficient to produce a complex network, and could be localized only at the tips of hyphae. However, branching can be of two types, apical or lateral, depending on its location on the hyphae, therefore imposing the redistribution of the necessary material in the whole mycelium. From an evolutionary point of view, maintaining different branching processes, with additional energy needs for structure and metabolism, is intriguing. We propose in this work to discuss the advantages of each branching type using a new observable for the network growth, allowing us to compare growth configurations. For this purpose, we build on experimental observations of the Podospora anserina mycelium growth, enabling us to feed and constrain a lattice-free modeling of this network based on a binary tree. First, we report the set of statistics related to the branches of P. anserina that we have implemented into the model. Then, we build the density observable, allowing us to discuss the succession of growth phases. We predict that density over time is not monotonic, but shows a decay growth phase, clearly separated from an other one by a stationary phase. The time of appearance of this stable region appears to be driven solely by the growth rate. Finally, we show that density is an appropriate observable to differentiate growth stress.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Dawkins, R. The evolution of evolvability. In Artificial Life 201–220 (Routledge, 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

