PD-1 blockade potentiates neoadjuvant chemotherapy in NSCLC via increasing CD127+ and KLRG1+ CD8 T cells
- PMID: 37231145
- PMCID: PMC10213055
- DOI: 10.1038/s41698-023-00384-x
PD-1 blockade potentiates neoadjuvant chemotherapy in NSCLC via increasing CD127+ and KLRG1+ CD8 T cells
Abstract
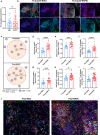
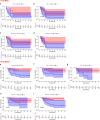
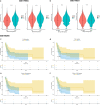
The combination of PD-1 blockade with neoadjuvant chemotherapy (NAC) has achieved unprecedented clinical success in non-small cell lung cancer (NSCLC) compared to NAC alone, but the underlying mechanisms by which PD-1 blockade augments the effects of chemotherapy remain incompletely elucidated. Single-cell RNA sequencing was performed on CD45+ immune cells isolated from surgically resected fresh tumors of seven NSCLC patients receiving NAC or neoadjuvant pembrolizumab and chemotherapy (NAPC). Multiplex fluorescent immunohistochemistry was performed on FFPE tissues before and after NAC or NAPC from 65 resectable NSCLC patients, and results were validated with GEO dataset. NAC resulted in an increase only of CD20+ B cells, whereas NAPC increased the infiltration of CD20+ B cells, CD4+ T cells, CD4+CD127+ T cells, CD8+ T cells, CD8+CD127+ and CD8+KLRG1+ T cells. Synergistic increase in B and T cells promotes favorable therapeutic response after NAPC. Spatial distribution analysis discovered that CD8+ T cells and their CD127+ and KLRG1+ subsets were in closer proximity to CD4+ T/CD20+ B cells in NAPC versus NAC. GEO dataset validated that B-cell, CD4, memory, and effector CD8 signatures correlated with therapeutic responses and clinical outcomes. The addition of PD-1 blockade to NAC promoted anti-tumor immunity through T and B cells recruitment in the tumor microenvironment and induced tumor-infiltrating CD8+ T cells skewed toward CD127+ and KLRG1+ phenotypes, which may be assisted by CD4+ T cells and B cells. Our comprehensive study identified key immune cell subsets exerting anti-tumor responses during PD-1 blockade therapy and that may be therapeutically targeted to improve upon existing immunotherapies for NSCLC.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: a Cancer J. Clin. 2022;72:7–33. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

