Structural basis for pre-tRNA recognition and processing by the human tRNA splicing endonuclease complex
- PMID: 37231153
- PMCID: PMC10627149
- DOI: 10.1038/s41594-023-00991-z
Structural basis for pre-tRNA recognition and processing by the human tRNA splicing endonuclease complex
Erratum in
-
Author Correction: Structural basis for pre-tRNA recognition and processing by the human tRNA splicing endonuclease complex.Nat Struct Mol Biol. 2024 Feb;31(2):390. doi: 10.1038/s41594-024-01213-w. Nat Struct Mol Biol. 2024. PMID: 38196035 No abstract available.
Abstract
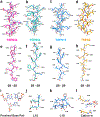
Throughout bacteria, archaea and eukarya, certain tRNA transcripts contain introns. Pre-tRNAs with introns require splicing to form the mature anticodon stem loop. In eukaryotes, tRNA splicing is initiated by the heterotetrameric tRNA splicing endonuclease (TSEN) complex. All TSEN subunits are essential, and mutations within the complex are associated with a family of neurodevelopmental disorders known as pontocerebellar hypoplasia (PCH). Here, we report cryo-electron microscopy structures of the human TSEN-pre-tRNA complex. These structures reveal the overall architecture of the complex and the extensive tRNA binding interfaces. The structures share homology with archaeal TSENs but contain additional features important for pre-tRNA recognition. The TSEN54 subunit functions as a pivotal scaffold for the pre-tRNA and the two endonuclease subunits. Finally, the TSEN structures enable visualization of the molecular environments of PCH-causing missense mutations, providing insight into the mechanism of pre-tRNA splicing and PCH.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests
The authors declare no conflict of interest.
Figures








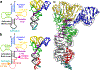

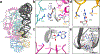

References
Methods-only references:
Publication types
MeSH terms
Substances
Grants and funding
- ZIC ES103326/ImNIH/Intramural NIH HHS/United States
- K99 GM143573/GM/NIGMS NIH HHS/United States
- ZIA ES103247/ImNIH/Intramural NIH HHS/United States
- ZIC ES103206/ImNIH/Intramural NIH HHS/United States
- R00 GM143534/GM/NIGMS NIH HHS/United States
- Z01 ES102487/ImNIH/Intramural NIH HHS/United States
- Z01 ES102488/ImNIH/Intramural NIH HHS/United States
- Z01 ES043010/ImNIH/Intramural NIH HHS/United States
- ZIC ES102488/ImNIH/Intramural NIH HHS/United States
- R35 GM136435/GM/NIGMS NIH HHS/United States
- ZIC ES102487/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources

