Immune Responses 6 Months After mRNA-1273 COVID-19 Vaccination and the Effect of a Third Vaccination in Patients with Inborn Errors of Immunity
- PMID: 37231290
- PMCID: PMC10212732
- DOI: 10.1007/s10875-023-01514-7
Immune Responses 6 Months After mRNA-1273 COVID-19 Vaccination and the Effect of a Third Vaccination in Patients with Inborn Errors of Immunity
Abstract
Purpose: Patients with inborn errors of immunity (IEI) are at increased risk of severe coronavirus disease-2019 (COVID-19). Effective long-term protection against COVID-19 is therefore of great importance in these patients, but little is known about the decay of the immune response after primary vaccination. We studied the immune responses 6 months after two mRNA-1273 COVID-19 vaccines in 473 IEI patients and subsequently the response to a third mRNA COVID-19 vaccine in 50 patients with common variable immunodeficiency (CVID).
Methods: In a prospective multicenter study, 473 IEI patients (including X-linked agammaglobulinemia (XLA) (N = 18), combined immunodeficiency (CID) (N = 22), CVID (N = 203), isolated or undefined antibody deficiencies (N = 204), and phagocyte defects (N = 16)), and 179 controls were included and followed up to 6 months after two doses of the mRNA-1273 COVID-19 vaccine. Additionally, samples were collected from 50 CVID patients who received a third vaccine 6 months after primary vaccination through the national vaccination program. SARS-CoV-2-specific IgG titers, neutralizing antibodies, and T cell responses were assessed.
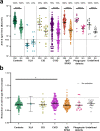
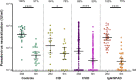
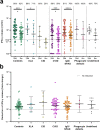
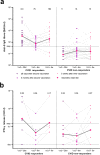
Results: At 6 months after vaccination, the geometric mean antibody titers (GMT) declined in both IEI patients and healthy controls, when compared to GMT 28 days after vaccination. The trajectory of this decline did not differ between controls and most IEI cohorts; however, antibody titers in CID, CVID, and isolated antibody deficiency patients more often dropped to below the responder cut-off compared to controls. Specific T cell responses were still detectable in 77% of controls and 68% of IEI patients at 6 months post vaccination. A third mRNA vaccine resulted in an antibody response in only two out of 30 CVID patients that did not seroconvert after two mRNA vaccines.
Conclusion: A similar decline in IgG titers and T cell responses was observed in patients with IEI when compared to healthy controls 6 months after mRNA-1273 COVID-19 vaccination. The limited beneficial benefit of a third mRNA COVID-19 vaccine in previous non-responder CVID patients implicates that other protective strategies are needed for these vulnerable patients.
Keywords: Inborn errors of immunity; SARS-CoV-2; T cell response; antibody response; immunogenicity; mRNA-1273 COVID-19 vaccine; primary immunodeficiency disorders.
© 2023. The Author(s).
Conflict of interest statement
JP received a grant from GlaxoSmithKline for an improvement of clinical care project and received support from Prothva Biosolutions for attending meetings and cover of travel expenses. JP participates in an Advisory Board for Janssen. FV received a grant from ZonMW for a study on lanadelumab in COVID-19, and consulting fees from GSK made to his department. VD received consulting fees from GlaxoSmithKline, Pharming NV for Advisory board meetings and honoraria for lectures from Takeda Pharmaceutical Company, Kedrion, AstraZeneca. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

