A new continuous noninvasive finger cuff device (Vitalstream) for cardiac output that communicates wirelessly via bluetooth or Wi-Fi
- PMID: 37231335
- PMCID: PMC10210402
- DOI: 10.1186/s12871-023-02114-z
A new continuous noninvasive finger cuff device (Vitalstream) for cardiac output that communicates wirelessly via bluetooth or Wi-Fi
Abstract
Background: The new noninvasive Vitalstream (VS) continuous physiological monitor (Caretaker Medical LLC, Charlottesville, Virginia), allows continuous cardiac output by a low pump-inflated, finger cuff that pneumatically couples arterial pulsations via a pressure line to a pressure sensor for detection and analysis. Physiological data are communicated wirelessly to a tablet-based user interface via Bluetooth or Wi-Fi. We evaluated its performance against thermodilution cardiac output in patients undergoing cardiac surgery.
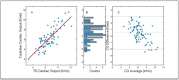
Methods: We compared the agreement between thermodilution cardiac output to that obtained by the continuous noninvasive system during cardiac surgery pre and post-cardiac bypass. Thermodilution cardiac output was performed routinely when clinically indicated by an iced saline cold injectate system. All comparisons between VS and TD/CCO data were post-processed. In order to match the VS CO readings to the averaged discrete TD bolus data, the averaged CO readings of the ten seconds of VS CO data points prior to a sequence of TD bolus injections was matched. Time alignment was based on the medical record time and the VS time-stamped data points. The accuracy against reference TD measurements was assessed via Bland-Altman analysis of the CO values and standard concordance analysis of the ΔCO values (with a 15% exclusion zone).
Results: Analysis of the data compared the accuracy of the matched measurement pairs of VS and TD/CCO VS absolute CO values with and without initial calibration to the discrete TD CO values, as well as the trending ability, i.e., ΔCO values of the VS physiological monitor compared to those of the reference. The results were comparable with other non-invasive as well as invasive technologies and Bland-Altman analyses showed high agreement between devices in a diverse patient population. The results are significant regarding the goal of expanding access to effective, wireless and readily implemented fluid management monitoring tools to hospital sections previously not covered because of the limitations of traditional technologies.
Conclusion: This study demonstrated that the agreement between the VS CO and TD CO was clinically acceptable with a percent error (PE) of 34.5 to 38% with and without external calibration. The threshold for an acceptable agreement between the VS and TD was considered to be below 40% which is below the threshold recommended by others.
Keywords: Agreement; Cardiac output; Cardiac surgery; Finger cuff; Non-invasive; Thermodilution; Wi-Fi Wireless device.
© 2023. The Author(s).
Conflict of interest statement
M.B. declares his employment with the funder of this study, Caretaker Medical, Charlottesville, VA. The remaining authors declare that they have no competing interests.
Figures




References
-
- Wagner JY, Grond J, Fortin J, Negulescu I, Schöfthaler M, Saugel B. Continuous noninvasive cardiac output determination using the CNAP System: evaluation of a Cardiac output algorithm for the analysis of volume clamp method-derived pulse contour. J Clin Monit Comput. 2016;30(4):487–93. 10.1007/s10877-015-9744-1. - PubMed
-
- Saugel B, Hoppe P, Nicklas JY, Kouz K, Körner A, Hempel JC, Jaap J, Vos G, Schön, Thomas WL, Scheeren. Continuous noninvasive pulse Wave Analysis using finger Cuff Technologies for arterial blood pressure and cardiac output monitoring in Perioperative and Intensive Care Medicine: a systematic review and Meta-analysis. Br J Anaesth. 2020;125(1):25–37. 10.1016/j.bja.2020.03.013. - PubMed
-
- Solà J, Delgado-Gonzalo R. The handbook of Cuffless Blood pressure monitoring: a practical guide for Clinicians, Researchers, and engineers. Springer Nature; 2019.

