Molecular and Functional Heterogeneity of Primary Pancreatic Neuroendocrine Tumors and Metastases
- PMID: 37232011
- PMCID: PMC10614458
- DOI: 10.1159/000530968
Molecular and Functional Heterogeneity of Primary Pancreatic Neuroendocrine Tumors and Metastases
Abstract
Introduction: Treatment response to the standard therapy is low for metastatic pancreatic neuroendocrine tumors (PanNETs) mainly due to the tumor heterogeneity. We investigated the heterogeneity between primary PanNETs and metastases to improve the precise treatment.
Methods: The genomic and transcriptomic data of PanNETs were retrieved from the Genomics, Evidence, Neoplasia, Information, Exchange (GENIE), and Gene Expression Omnibus (GEO) database, respectively. Potential prognostic effects of gene mutations enriched in metastases were investigated. Gene set enrichment analysis was performed to investigate the functional difference. Oncology Knowledge Base was interrogated for identifying the targetable gene alterations.
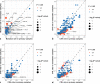
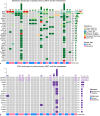
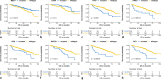
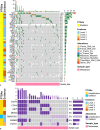
Results: Twenty-one genes had significantly higher mutation rates in metastases which included TP53 (10.3% vs. 16.9%, p = 0.035) and KRAS (3.7% vs. 9.1%, p = 0.016). Signaling pathways related to cell proliferation and metabolism were enriched in metastases, whereas epithelial-mesenchymal transition (EMT) and TGF-β signaling were enriched in primaries. Gene mutations were highly enriched in metastases that had significant unfavorable prognostic effects included mutation of TP53 (p < 0.001), KRAS (p = 0.001), ATM (p = 0.032), KMT2D (p = 0.001), RB1 (p < 0.001), and FAT1 (p < 0.001). Targetable alterations enriched in metastases included mutation of TSC2 (15.5%), ARID1A (9.7%), KRAS (9.1%), PTEN (8.7%), ATM (6.4%), amplification of EGFR (6.0%), MET (5.5%), CDK4 (5.5%), MDM2 (5.0%), and deletion of SMARCB1 (5.0%).
Conclusion: Metastases exhibited a certain extent of genomic and transcriptomic diversity from primary PanNETs. TP53 and KRAS mutation in primary samples might associate with metastasis and contribute to a poorer prognosis. A high fraction of novel targetable alterations enriched in metastases deserves to be validated in advanced PanNETs.
Keywords: Genetics; Metastasis; Molecular heterogeneity; Pancreatic neuroendocrine tumors; Targeted therapy.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures


References
-
- Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018 Dec;31(12):1770–86. 10.1038/s41379-018-0110-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

